了解离子液体介导的溶剂工程对苯丙氨酸氨解酶动力学和热力学稳定性的影响
IF 2.9
2区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
苯丙氨酸氨水解酶(PAL)在苯丙氨酸途径和治疗苯丙酮尿症中发挥着核心作用。然而,PAL 在苛刻条件下的不稳定性阻碍了其与可持续工业生物催化的整合。本研究证明,离子液体(IL)辅助溶剂(Tris-HCl 缓冲液)工程能够改善 Rhodotorula glutinisPAL(RgPAL)在各种压力下的反应动力学和热力学稳定性。在优化条件下,[Ch][Ac]混合物 Tris-HCl 溶剂中的 RgPAL 的 Kcat 值比原始 Tris-HCl 溶剂高 66.2%,室温储存 5 周后剩余活性为 60%,60 °C 孵育 1 h 后活性为 80%。光谱和分子对接结果表明,更高的水合程度和 IL 与 RgPAL 的 D 链残基之间的软相互作用共同促成了 RgPAL 更稳定和更活跃的构象。与原始的 Tris-HCl 相比,该酶在 ILs+Tris-HCl 中的熔化温度(Tm)更高,而解折焓(ΔHfu)和熵(ΔSfu)的变化较小。总之,IL 介导的溶剂工程改变了 RgPAL 的微环境,使基于 PAL 的稳健生物催化系统得以开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
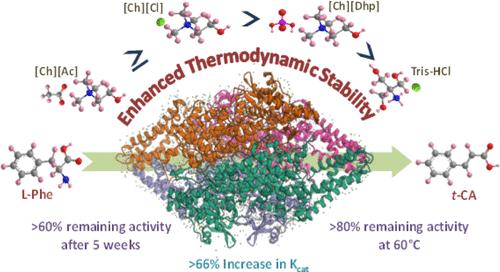
Understanding the Effect of Ionic Liquid–Mediated Solvent Engineering on the Kinetics and Thermodynamic Stability of Phenylalanine Ammonia-Lyase
Phenylalanine ammonia-lyase (PAL) plays a central role in the phenylpropanoid pathway and in the treatment of phenylketonuria. However, the integration of PAL into sustainable industrial biocatalysis is hampered by its instability under harsh conditions. This study demonstrates that ionic liquid (IL)–assisted solvent (Tris-HCl buffer) engineering enables improvement of the reaction kinetics and thermodynamic stability of Rhodotorula glutinisPAL (RgPAL) under various stresses. Under optimized conditions, a 66.2% higher Kcat value, >60% remaining activity after 5 weeks of storage at room temperature, and >80% activity of RgPAL after incubation at 60 °C for 1 h were obtained in the [Ch][Ac]-blended Tris-HCl solvent compared to pristine Tris-HCl. The spectroscopic and molecular docking results suggest that the higher extent of hydration and the soft interactions complemented by the ILs with the D-chain residues of RgPAL jointly contributed to achieving more stable and active conformations of RgPAL. The enzyme showed a higher melting temperature (Tm) in ILs+Tris-HCl compared to that in pristine Tris-HCl, with less change in enthalpy (ΔHfu) and entropy (ΔSfu) of unfolding. Overall, IL-mediated solvent engineering alters the microenvironment of RgPAL and allows the development of a robust PAL-based biocatalytic system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.80
自引率
9.10%
发文量
965
审稿时长
1.6 months
期刊介绍:
An essential criterion for acceptance of research articles in the journal is that they provide new physical insight. Please refer to the New Physical Insights virtual issue on what constitutes new physical insight. Manuscripts that are essentially reporting data or applications of data are, in general, not suitable for publication in JPC B.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


