胰蛋白酶将载脂蛋白 A-I (ApoA-I) 前体部分裂解为成熟的载脂蛋白 A-I,从而阻碍了液相色谱-多重反应监测模式质谱法(LC-MRM-MS)对天然存在的载脂蛋白 A-I 蛋白形态的定量分析
IF 3.1
2区 化学
Q2 BIOCHEMICAL RESEARCH METHODS
Journal of the American Society for Mass Spectrometry
Pub Date : 2024-09-20
DOI:10.1021/jasms.4c00155
引用次数: 0
摘要
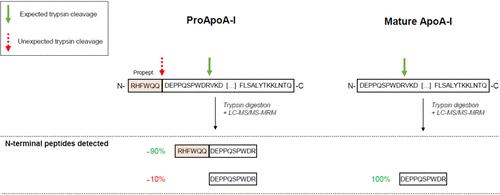
载脂蛋白 A-I(ApoA-I)是血浆中含量最高的蛋白质之一,也是高密度脂蛋白(HDL)的主要蛋白质成分。这两种载脂蛋白 A-I 蛋白形态在生物样本中共存,仅在 N 端有所不同。实际上,区分它们的唯一方法是使用液相色谱-多反应监测模式质谱(LC-MRM-MS)检测蛋白型特异的 N 端蛋白水解肽(分别为 RHFWQQDEPPQSPWDR 和 DEPPQSPWDR)。我们开发了一种自下而上的 LC-MRM-MS 方法,可同时检测原载脂蛋白 A-I 和成熟载脂蛋白 A-I。为了测试该方法的特异性,我们用胰蛋白酶消化了纯化的成熟 ApoA-I 和重组 proApoA-I。不出所料,消化成熟载脂蛋白 ApoA-I 时,只检测到与成熟载脂蛋白 ApoA-I 蛋白形式相对应的 N 端肽段 (DEPPQSPWDR)。然而,消化 proApoA-I 时不仅产生了与 proApoA-I 蛋白形式相对应的 N 端肽(RHFWQQDEPPQSPWDR),还产生了与成熟 ApoA-I 蛋白形式相对应的 N 端胰蛋白酶肽(DEPPQSPWDR)。标准胰蛋白酶和高特异性胰蛋白酶以及 Arg-C 酶都能以自我限制的方式产生这种效果(约占总量的 10%)。合成的 proApo-I 肽不会被胰蛋白酶裂解,这表明报告中的效应取决于蛋白质的构象。这种影响不容忽视,因为它可以通过 LC-MRM-MS 检测到,而且在这两种蛋白形式可能共存的生物样本中,应该应用校正计算来准确量化原载脂蛋白 ApoA-I 和成熟载脂蛋白 ApoA-I。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Trypsin Partially Cleaves Apolipoprotein A-I (ApoA-I) Precursor into Mature ApoA-I Hindering the Quantification of Naturally Occurring ApoA-I Proteoforms by Liquid Chromatography in Multiple Reaction Monitoring Mode Mass Spectrometry (LC-MRM-MS)
Apolipoprotein A-I (ApoA-I), one of the most abundant proteins in plasma and the major protein component of high-density lipoprotein (HDL), is naturally found in several proteoforms; two of them are ProApoA-I and mature ApoA-I. These two proteoforms of ApoA-I coexist in biological samples and differ only in their N-terminal end. Virtually, the only way to differentiate them is by detecting the proteoform-specific N-terminal proteolytic peptides (RHFWQQDEPPQSPWDR and DEPPQSPWDR, respectively) using liquid chromatography in multiple reaction monitoring mode mass spectrometry (LC-MRM-MS). We have developed a bottom-up LC-MRM-MS method to simultaneously detect proApoA-I and mature ApoA-I. To test the specificity of the method, we digested with trypsin purified mature ApoA-I and recombinant proApoA-I. As expected, only the N-term peptide corresponding to the mature ApoA-I proteoform (DEPPQSPWDR) was detected when digesting mature ApoA-I. However, the digestion of the proApoA-I produced not only the N-terminal peptide corresponding to proApoA-I (RHFWQQDEPPQSPWDR) but also the N-terminal tryptic peptide corresponding to mature ApoA-I (DEPPQSPWDR). This effect was produced by standard and high-specificity trypsin as well as by the Arg-C enzyme in a self-limited manner (approximately 10% of the total). The synthetic proApo-I peptide is not cleaved by trypsin, suggesting that the here reported effect is dependent on protein conformation. The effect is not negligible, as it can be detected by LC-MRM-MS, and correction calculations should be applied to accurately quantify proApoA-I and mature ApoA-I in biological samples where these two proteoforms may coexist.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.50
自引率
9.40%
发文量
257
审稿时长
1 months
期刊介绍:
The Journal of the American Society for Mass Spectrometry presents research papers covering all aspects of mass spectrometry, incorporating coverage of fields of scientific inquiry in which mass spectrometry can play a role.
Comprehensive in scope, the journal publishes papers on both fundamentals and applications of mass spectrometry. Fundamental subjects include instrumentation principles, design, and demonstration, structures and chemical properties of gas-phase ions, studies of thermodynamic properties, ion spectroscopy, chemical kinetics, mechanisms of ionization, theories of ion fragmentation, cluster ions, and potential energy surfaces. In addition to full papers, the journal offers Communications, Application Notes, and Accounts and Perspectives

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


