作为 HIV-1 蛋白酶抑制剂的 Darunavir 类似物的设计、合成和生物学评价
IF 3.8
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
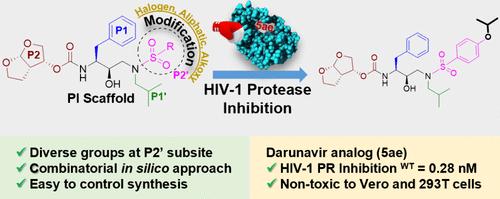
达芦那韦是治疗艾滋病病毒感染的一线药物,但由于新出现的多药耐药(MDR)艾滋病病毒株而面临局限性,因此有必要开发具有更高活性的类似物。在这项研究中,我们采用了一种组合硅学方法,初步设计了一系列在关键位点(P1′和P2′)进行修饰的HIV-1 PI类似物,以增强与HIV-1 PR的相互作用。我们选择了 15 种具有良好结合得分的类似物进行合成,并对其抑制 HIV-1 PR 的活性进行了评估。结果发现,P2′取代的变化是有效的,如 5aa (1.54 nM)、5ad (0.71 nM)、5ac (0.31 nM)、5ae (0.28 nM) 和 5af (1.12 nM),苯基亚砜基团上的卤素、脂肪族和烷氧基官能团对 HIV-1 PR 的抑制作用优于 DRV,在 Vero 和 293T 细胞系中观察到的细胞毒性最小。此外,计算研究表明,所选类似物具有抑制各种 HIV-1 PR 突变的潜力,包括 I54M 和 I84V。进一步的结构动力学和能量分析证实了有前景的类似物的稳定性和结合亲和力,尤其是 5ae,它与 HIV-1 PR 中的关键残基有很强的相互作用。总之,这项研究强调了灵活分子和增强 HIV-1 PR S2′位点相互作用对于开发有效的 DRV 类似物以抗击艾滋病毒和其他全球健康问题的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, Synthesis, and Biological Evaluation of Darunavir Analogs as HIV-1 Protease Inhibitors
Darunavir, a frontline treatment for HIV infection, faces limitations due to emerging multidrug resistant (MDR) HIV strains, necessitating the development of analogs with improved activity. In this study, a combinatorial in silico approach was used to initially design a series of HIV-1 PI analogs with modifications at key sites, P1′ and P2′, to enhance interactions with HIV-1 PR. Fifteen analogs with promising binding scores were selected for synthesis and evaluated for the HIV-1 PR inhibition activity. The variation of P2′ substitution was found to be effective, as seen in 5aa (1.54 nM), 5ad (0.71 nM), 5ac (0.31 nM), 5ae (0.28 nM), and 5af (1.12 nM), featuring halogen, aliphatic, and alkoxy functionalities on the phenyl sulfoxide motif exhibited superior inhibition against HIV-1 PR compared to DRV, with minimal cytotoxicity observed in Vero and 293T cell lines. Moreover, computational studies demonstrated the potential of selected analogs to inhibit various HIV-1 PR mutations, including I54M and I84V. Further structural dynamics and energetic analyses confirmed the stability and binding affinity of promising analogs, particularly 5ae, which showed strong interactions with key residues in HIV-1 PR. Overall, this study underscores the importance of flexible moieties and interaction enhancement at the S2′ subsite of HIV-1 PR in developing effective DRV analogs to combat HIV and other global health issues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Bio & Med Chem Au
药物、生物、化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Bio & Med Chem Au is a broad scope open access journal which publishes short letters comprehensive articles reviews and perspectives in all aspects of biological and medicinal chemistry. Studies providing fundamental insights or describing novel syntheses as well as clinical or other applications-based work are welcomed.This broad scope includes experimental and theoretical studies on the chemical physical mechanistic and/or structural basis of biological or cell function in all domains of life. It encompasses the fields of chemical biology synthetic biology disease biology cell biology agriculture and food natural products research nucleic acid biology neuroscience structural biology and biophysics.The journal publishes studies that pertain to a broad range of medicinal chemistry including compound design and optimization biological evaluation molecular mechanistic understanding of drug delivery and drug delivery systems imaging agents and pharmacology and translational science of both small and large bioactive molecules. Novel computational cheminformatics and structural studies for the identification (or structure-activity relationship analysis) of bioactive molecules ligands and their targets are also welcome. The journal will consider computational studies applying established computational methods but only in combination with novel and original experimental data (e.g. in cases where new compounds have been designed and tested).Also included in the scope of the journal are articles relating to infectious diseases research on pathogens host-pathogen interactions therapeutics diagnostics vaccines drug-delivery systems and other biomedical technology development pertaining to infectious diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


