肝细胞癌特异性表观遗传检查点双向调节 CD4 + T 细胞的抗肿瘤免疫力
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
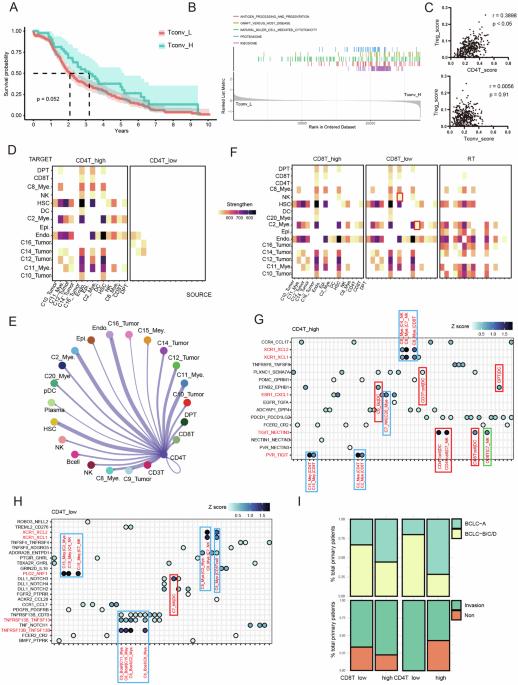
肝细胞癌(HCC)是一种对全球健康有重大影响的高度恶性肿瘤。CD4+ T细胞,尤其是常规CD4+ T细胞(Tconvs)在HCC进展中的作用仍未得到研究。此外,表观遗传因子在免疫调节中至关重要,但它们在 HCC 浸润的 Tconv 细胞中的具体作用仍不明确。本研究阐明了表观遗传调节因子 MATR3 在 HCC 微环境中调节 Tconv 活性和免疫逃避的作用。对 scRNA-seq 数据的重新分析表明,CD4+ T 细胞的早期激活对于建立抗肿瘤免疫反应至关重要。体内和体外实验显示,Tconv能增强cDC1诱导的CD8+ T细胞活化。筛选发现 MATR3 是 Tconv 功能的关键调节因子,它是抗肿瘤活性所必需的,但过度表达则有害。MATR3 的过度表达会加剧 Tconv 的衰竭,并通过招募 SWI/SNF 复合物松弛 TOX 启动子区域的染色质来损害其功能,从而导致异常的转录变化。总之,MATR3是一种HCC特异性表观遗传检查点,它能双向调节Tconv的抗肿瘤免疫功能,这提示了针对表观遗传调节因子的新治疗策略,以增强HCC的抗肿瘤免疫功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hepatocellular carcinoma-specific epigenetic checkpoints bidirectionally regulate the antitumor immunity of CD4 + T cells
Hepatocellular carcinoma (HCC) is a highly malignant tumor with significant global health implications. The role of CD4+ T cells, particularly conventional CD4+ T cells (Tconvs), in HCC progression remains unexplored. Furthermore, epigenetic factors are crucial in immune regulation, yet their specific role in HCC-infiltrating Tconv cells remains elusive. This study elucidates the role of MATR3, an epigenetic regulator, in modulating Tconv activity and immune evasion within the HCC microenvironment. Reanalysis of the scRNA-seq data revealed that early activation of CD4+ T cells is crucial for establishing an antitumor immune response. In vivo and in vitro experiments revealed that Tconv enhances cDC1-induced CD8+ T-cell activation. Screening identified MATR3 as a critical regulator of Tconv function, which is necessary for antitumour activity but harmful when overexpressed. Excessive MATR3 expression exacerbates Tconv exhaustion and impairs function by recruiting the SWI/SNF complex to relax chromatin in the TOX promoter region, leading to aberrant transcriptional changes. In summary, MATR3 is an HCC-specific epigenetic checkpoint that bidirectionally regulates Tconv antitumour immunity, suggesting new therapeutic strategies targeting epigenetic regulators to enhance antitumour immunity in HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


