DDX5 通过维持 VAV3 mRNA 的稳定性促进食管鳞状细胞癌的生长
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
食管鳞状细胞癌(ESCC)的预防和治疗迫切需要新的治疗靶点及其抑制剂。本研究旨在研究DEAD-box螺旋酶5(DDX5)在ESCC进展中的功能,并找出一种有前景的DDX5抑制剂。我们证实,DDX5在ESCC中高表达,并发挥致癌作用,它与VAV鸟嘌呤核苷酸交换因子3(VAV3)mRNA结合,并通过与m6A甲基转移酶3(METTL3)相互作用促进VAV3 mRNA的N6-甲基腺苷(m6A)修饰。经 M6A 修饰的 VAV3 mRNA 被胰岛素样生长因子 1(IGF2BP1)识别,从而增加了 mRNA 的稳定性。甲基尼索林-3-β-D-O-葡萄糖苷(MD)通过DDX5-VAV3轴抑制了ESCC的进展。我们的研究结果表明,DDX5会促进ESCC的进展。MD通过靶向DDX5抑制ESCC的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
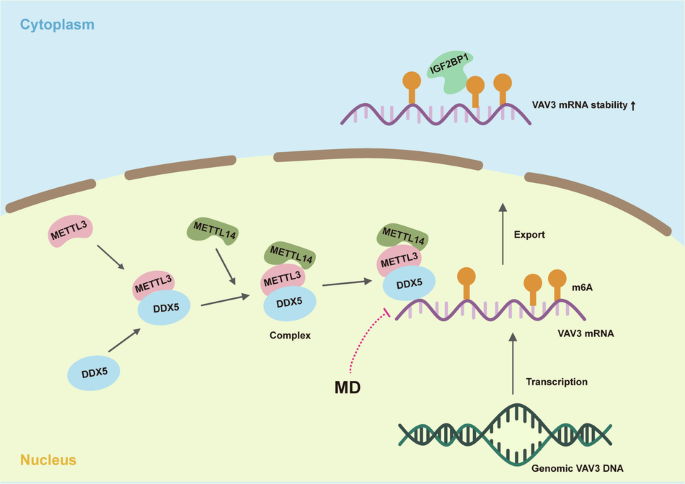
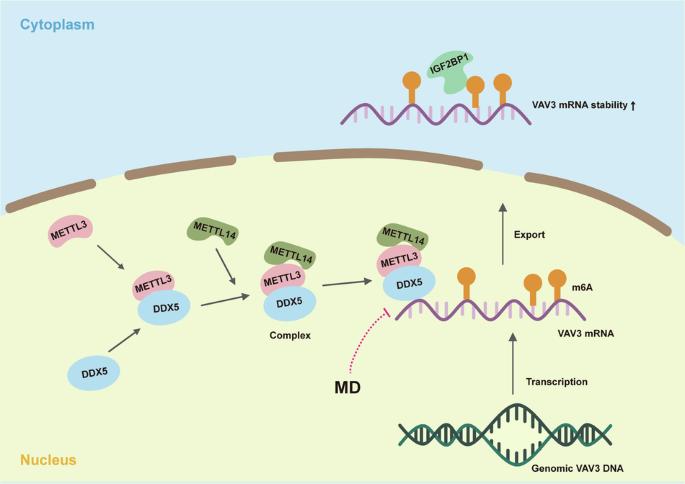
DDX5 promotes esophageal squamous cell carcinoma growth through sustaining VAV3 mRNA stability
Novel therapeutic targets and their inhibitors for esophageal squamous cell carcinoma (ESCC) prevention and therapy are urgently needed. This study aimed to investigate the function of DEAD-box helicase 5 (DDX5) in ESCC progression and to identify a promising inhibitor of DDX5. We verified that DDX5 was highly expressed in ESCC and played an oncogenic role, binding with vav guanine nucleotide exchange factor 3 (VAV3) mRNA and facilitating VAV3 mRNA N6-methyladenosine (m6A) modification by interacting with the m6A methyltransferase 3 (METTL3). M6A-modified VAV3 mRNA was identified by insulin-like growth factor 1 (IGF2BP1), increasing mRNA stability. Methylnissolin-3-β-D-O-glucoside (MD) inhibited ESCC progression through the DDX5-VAV3 axis. Our findings suggest that DDX5 promotes ESCC progression. MD inhibits ESCC progression by targeting DDX5.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


