RNA 螺旋酶 DDX21 在结直肠癌中激活 YAP 促进肿瘤发生,并受β-catenin 的转录上调作用
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
RNA螺旋酶DDX21对核糖体的生物发生至关重要,并在CRC中上调,但DDX21的失调机制以及DDX21促进CRC肿瘤发生的机制仍不甚明了。在这里,我们发现DDX21是β-catenin的直接转录靶基因,并介导了β-catenin在CRC中的原发性致瘤功能。DDX21 的表达与 β-catenin 的表达和活性相关,DDX21 的高表达与 CRC 患者的不良预后相关。DDX21的缺失会导致YAP的胞质转移和转录活性降低,并抑制CRC细胞的增殖和迁移,而YAP的重新激活可以部分缓解这种情况。重要的是,通过使用翻译延伸抑制剂和DNA插入因子,我们发现核糖体应激可上调DDX21的表达,并诱导LATS下调和YAP活化,这可能是通过ZAKα-MKK4/7-JNK轴实现的。总之,我们的研究揭示了 DDX21 在 CRC 中的转录激活机制以及 YAP 在核糖体应激反应中的激活机制,这表明包括诱导核糖体应激和抑制 YAP 的联合疗法具有潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
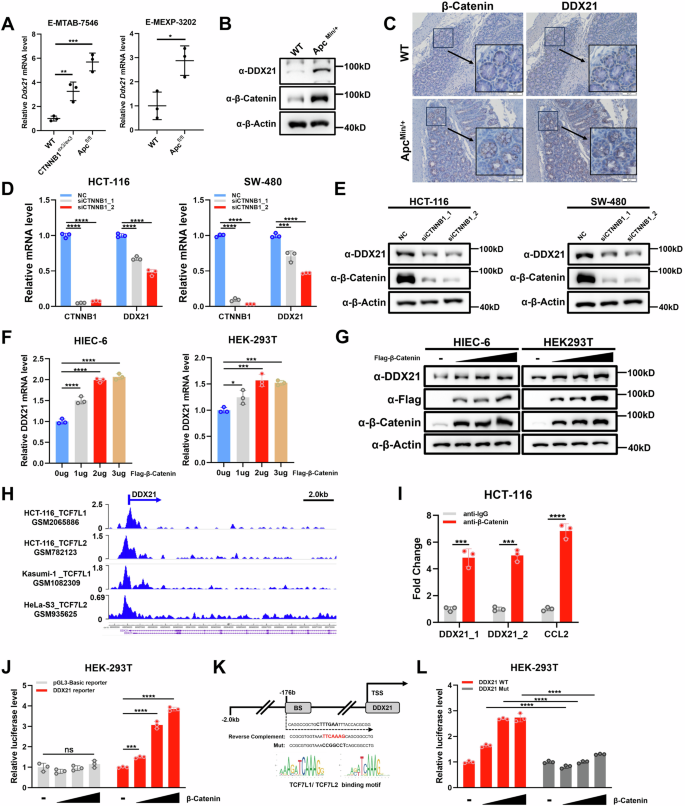
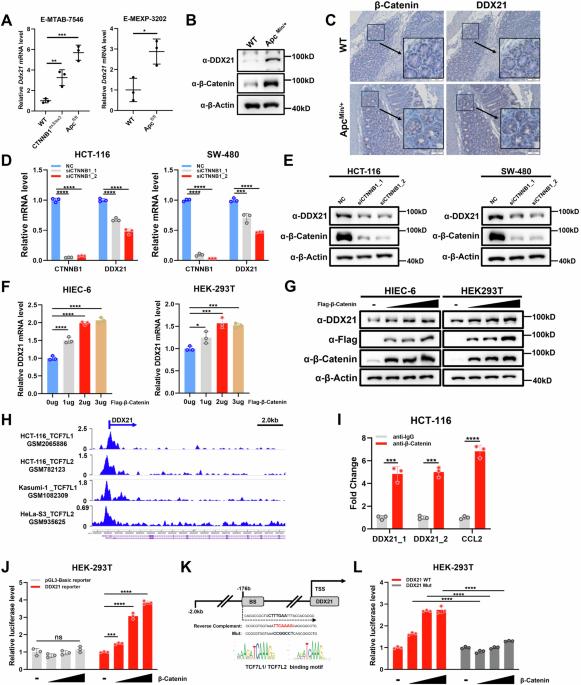
The RNA helicase DDX21 activates YAP to promote tumorigenesis and is transcriptionally upregulated by β-catenin in colorectal cancer
The RNA helicase DDX21 is vital for ribosome biogenesis and is upregulated in CRC, but the mechanism by which DDX21 is dysregulated and by which DDX21 promotes tumorigenesis in CRC remains poorly understood. Here, we showed that DDX21 is a direct transcriptional target gene of β-catenin and mediates the protumorigenic function of β-catenin in CRC. DDX21 expression is correlated with the expression and activity of β-catenin, and high DDX21 expression is associated with a poor prognosis in CRC patients. Loss of DDX21 leads to cytoplasmic translocation and decreased transcriptional activity of YAP and suppresses the proliferation and migration of CRC cells, which can be partially rescued by YAP reactivation. Importantly, by using translation elongation inhibitors and DNA intercalators, we showed that ribosomal stress upregulates DDX21 expression and induces the downregulation of LATS and the activation of YAP, probably through the ZAKα-MKK4/7-JNK axis. Overall, our study revealed the transcriptional activation mechanism of DDX21 in CRC and the activation of YAP in the ribosomal stress response, indicating the potential of combination therapy involving the induction of ribosomal stress and YAP inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


