基于肿瘤细胞外囊泡的紫杉醇递送系统在肝癌中的化学免疫治疗作用
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
作为一线化疗药物,白蛋白结合型紫杉醇(PA)对各种癌症的治疗具有显著效果。然而,在肝癌化疗中,由于肝癌细胞的先天耐药性,对紫杉醇的敏感性低,毒副作用大,临床治疗效果差。在这项研究中,我们提出了一种独特的纳米给药系统。通过差速离心法分离纯化了紫外线(UV)诱导的肿瘤细胞源性细胞外囊泡(EVs)。然后,通过共挤技术将 PA 加入到囊泡中,制成囊泡药物递送系统(EVPA)。利用EV依赖性增强的滞留效应和特异性归巢效应,EVPA可被动和主动地靶向肿瘤组织,激活免疫反应释放PA,实现肝癌化疗和免疫治疗的联合治疗效果。我们在体内和体外都证明了EVPA的杀瘤效果优于PA,而且EVPA能被肝癌细胞和树突状细胞有效摄取,激活机体的特异性免疫反应,促进CD4+和CD8+T细胞在肿瘤组织中的浸润,通过化学免疫疗法对肝癌细胞发挥精确的杀伤作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
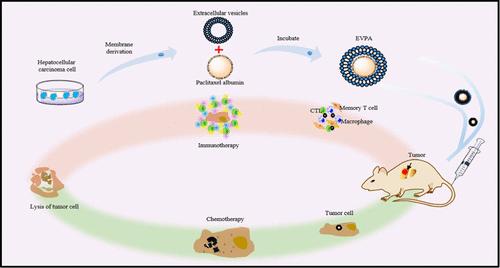
The Chemo-Immunotherapeutic Roles of Tumor-Derived Extracellular Vesicle-Based Paclitaxel Delivery System in Hepatocarcinoma
As a first-line chemotherapeutic agent, albumin-bound paclitaxel (PA) has a considerable effect on the treatment of various cancers. However, in chemotherapy for hepatocarcinoma, the sensitivity to PA is low owing to the innate resistance of hepatocarcinoma cells; the toxicity and side effects are severe, and the clinical treatment impact is poor. In this study, we present a unique nanodrug delivery system. The ultraviolet (UV)-induced tumor-cell-derived extracellular vesicles (EVs) were isolated and purified by differential centrifugation. Then, PA was loaded by coextrusion to create a vesicle drug delivery system (EVPA). By employing the EV-dependent enhanced retention effect and specific homing effect, EVPA would passively and actively target tumor tissues, activate the immune response to release PA, and achieve the combination therapeutic effect of chemo-immunotherapy on hepatocarcinoma. We demonstrated that the tumor-killing effect of EVPA is superior to that of PA, both in vivo and in vitro and that EVPA can be effectively taken up by hepatocarcinoma and dendritic cells, activate the body’s specific immune response, promote the infiltration of CD4+ and CD8+ T cells in tumor tissues, and exert a precise killing effect on hepatocarcinoma cells via chemo-immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


