与葡萄糖基纳米球结合的脂结合还原氟哌啶醇:胶质瘤治疗策略
IF 4.5
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
侵袭性胶质瘤的生存率很低。肿瘤侵袭性的增加与肿瘤细胞和肿瘤相关巨噬细胞(TAMs)都有关系,后者会诱发肿瘤的侵袭、入侵和转移。因此,有效的治疗必须同时针对胶质瘤细胞和 TAMs。氟哌啶醇是一种神经精神类药物,可同时靶向两种细胞中表达水平较高的σ受体(SR)。在此,我们介绍了一种新型阳离子脂质共轭还原型氟哌啶醇(±RHPC8)的开发情况,其目的是介导以SR为靶点的抗胶质瘤效应。假设±RHPC8可同时作为SR靶向配体和抗癌剂。由于血脑屏障(BBB)阻碍了对原位胶质瘤的直接靶向治疗,因此我们使用可穿过BBB的葡萄糖基碳纳米球(CSPs)将±RHPC8送入胶质瘤肿瘤小鼠脑内。由此产生的±RHPC8-CSP纳米共轭物靶向表达SR的胶质瘤细胞。在小鼠正位和皮下肿瘤模型中,与其他治疗组相比,±RHPC8-CSP可延长生存期并使肿瘤消退。值得注意的是,±RHPC8-CSP能被SR表达的TAMs明显吸收,从而导致巨噬细胞从M2极化到M1,这表现在TAMs释放的免疫抑制细胞因子(包括TGF-β、IL-10和VEGF)的表达明显减少。总之,所设计的±RHPC8-CSP 纳米共轭物是治疗脑癌的有效纳米药物递送系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
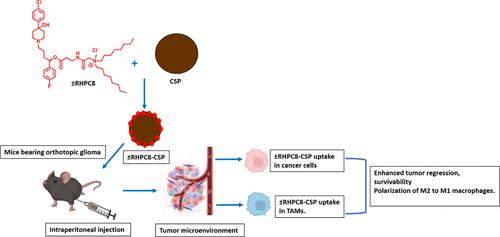
Lipid-Conjugated Reduced Haloperidol in Association with Glucose-Based Nanospheres: A Strategy for Glioma Treatment
Aggressive glioma exhibits a poor survival rate. Increased tumor aggression is linked to both tumor cells and tumor-associated macrophages (TAMs), which induce pro-aggression, invasion, and metastasis. Imperatively, for effective treatment, it is important to target both glioma cells and TAMs. Haloperidol, a neuropsychotic drug, avidly targets the sigma receptor (SR), which is expressed in higher levels in both the cell types. Herein, we present the development of a novel cationic lipid-conjugated reduced haloperidol (±RHPC8), which aims to mediate the SR-targeted antiglioma effect. Hypothetically, ±RHPC8 would act simultaneously as an SR-targeting ligand and anticancer agent. As the blood–brain barrier (BBB) obstructs direct targeting of in situ glioma, we used BBB-crossing glucose-based carbon nanospheres (CSPs) to deliver ±RHPC8 within the glioma tumor-bearing mouse brain. The resultant ±RHPC8-CSP nanoconjugate targeted SR-expressing glioma cells. In both orthotopic and subcutaneous mouse tumor models, ±RHPC8-CSP prolonged survival and regressed tumors compared to other treated groups. Notably, ±RHPC8-CSP was significantly taken up by SR-expressing TAMs thus resulting in macrophage polarization from M2 to M1, as exhibited by markedly reduced expression of immunosuppressive cytokines released by TAMs, including TGF-β, IL-10, and VEGF. In conclusion, the designed ±RHPC8-CSP nanoconjugate presented an effective nanodrug delivery system for brain cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Pharmaceutics
医学-药学
CiteScore
8.00
自引率
6.10%
发文量
391
审稿时长
2 months
期刊介绍:
Molecular Pharmaceutics publishes the results of original research that contributes significantly to the molecular mechanistic understanding of drug delivery and drug delivery systems. The journal encourages contributions describing research at the interface of drug discovery and drug development.
Scientific areas within the scope of the journal include physical and pharmaceutical chemistry, biochemistry and biophysics, molecular and cellular biology, and polymer and materials science as they relate to drug and drug delivery system efficacy. Mechanistic Drug Delivery and Drug Targeting research on modulating activity and efficacy of a drug or drug product is within the scope of Molecular Pharmaceutics. Theoretical and experimental peer-reviewed research articles, communications, reviews, and perspectives are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


