肝细胞癌的肿瘤靶向 CO 纳米输送系统设计与疗法
IF 4.5
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
近年来,一氧化碳(CO)作为一种治疗肝细胞癌(HCC)的新型绿色疗法受到越来越多的关注。然而,由于一氧化碳供体缺乏靶向能力且释放率不稳定,其临床应用仍然受到限制。本文首先设计了葡萄糖-聚乙二醇(PEG)-硫辛酸(LA)-Fe2(CO)6(Glu-Fe2(CO)6)自组装两亲纳米细胞作为CO供体,并通过化学方法合成,通过PEG-LA将葡萄糖与Fe2(CO)6结合在一起。这种肿瘤靶向 Glu-Fe2(CO)6 递送系统的优点包括:(I) 良好的水溶性;(II) 谷胱甘肽响应性 CO 缓释;(III) 葡萄糖作为靶向配体的肿瘤靶向活性;(IV) 在体外和体内对 HCC 的抗肿瘤疗效和 CO 治疗的安全性。这些研究结果表明,Glu-Fe2(CO)6 纳米微球有望提高抗肿瘤治疗能力,为气体治疗 HCC 提供了一种新型的肿瘤靶向递送策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
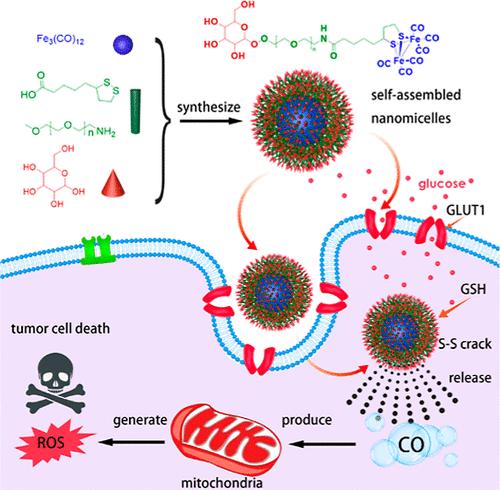
Tumor-Targeted CO Nanodelivery System Design and Therapy for Hepatocellular Carcinoma
In recent years, carbon monoxide (CO) has garnered increased attention as a novel green therapy for hepatocellular carcinoma (HCC) treatment. However, the CO donor is still limited in clinical application due to its lack of targeted ability and unstable release rate. Here, self-assembled amphiphilic nanomicelles glucose-polyethylene glycol (PEG)–lipoic acid (LA)–Fe2(CO)6 (Glu-Fe2(CO)6) are first designed as a CO donor and synthesized via a chemical method, combining glucose with Fe2(CO)6 through PEG–LA. Some advantages of this tumor-targeted Glu-Fe2(CO)6 delivery system include (I) good water-solubility, (II) the glutathione responsive CO slow release, (III) the active tumor-targeted ability of glucose as targeted ligands, and (IV) outstanding efficacy of antitumor and safety of CO therapy of HCC both in vitro and in vivo. These findings suggest that Glu-Fe2(CO)6 nanomicelles hold promise for enhancing antitumor therapeutic capabilities, presenting a novel tumor-targeted delivery strategy in gas therapy for HCC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Pharmaceutics
医学-药学
CiteScore
8.00
自引率
6.10%
发文量
391
审稿时长
2 months
期刊介绍:
Molecular Pharmaceutics publishes the results of original research that contributes significantly to the molecular mechanistic understanding of drug delivery and drug delivery systems. The journal encourages contributions describing research at the interface of drug discovery and drug development.
Scientific areas within the scope of the journal include physical and pharmaceutical chemistry, biochemistry and biophysics, molecular and cellular biology, and polymer and materials science as they relate to drug and drug delivery system efficacy. Mechanistic Drug Delivery and Drug Targeting research on modulating activity and efficacy of a drug or drug product is within the scope of Molecular Pharmaceutics. Theoretical and experimental peer-reviewed research articles, communications, reviews, and perspectives are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


