通过臭氧低温模板氧化提高沸石的酸度窗口
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
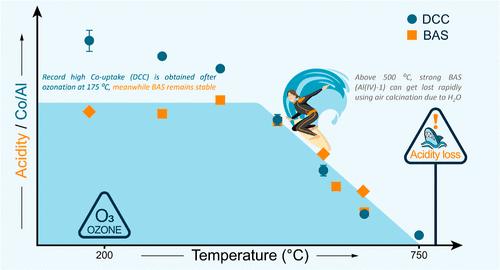
重新审视第一个通常被认为是微不足道的后合成步骤(即高温氧化煅烧以去除有机模板)的影响,增加了我们对热酸性位点演变和铝分布的了解。通过使用低温臭氧方法,对高硅沸石(SSZ-13)的酸度实现了前所未有的控制。吸附探针分子的傅立叶变换红外光谱和固态核磁共振光谱揭示了酸性位点热演化的复杂性。低温活化(臭氧)沸石保持了原有的布氏酸度和高缺陷含量,几乎没有路易斯酸度。它们还保持了结晶后 "原样 "的铝分布,并显示出合成条件与二价阳离子容量之间的明显联系,这是用水溶液钴离子吸收量来衡量的。合成方案是导致铝接近的主要因素,在臭氧处理时可产生创纪录的高交换容量。然而,在 500-600 °C 的传统煅烧温度下,水的存在会导致布氏酸位点逐渐耗尽,尤其是在小晶体中。这项研究表明,低温臭氧处理后在不同温度下进行热活化可作为一种新工具,用于调整酸性位点的数量和性质,从而深入了解沸石在酸催化反应(如二氧化碳加氢制二甲醚)中的活性,并由此拓展合理调整酸度的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing the Acidity Window of Zeolites by Low-Temperature Template Oxidation with Ozone
Revisiting the impact of the first and often deemed trivial postsynthetic step, i.e., a high-temperature oxidative calcination to remove organic templates, increases our understanding of thermal acid site evolution and Al distributions. An unprecedented degree of control over the acidity of high-silica zeolites (SSZ-13) was achieved by using a low-temperature ozonation approach. Fourier transform infrared spectroscopy of adsorbed probe molecules and solid-state NMR spectroscopy reveal the complexity of the thermal evolution of acid sites. Low-temperature activated (ozonated) zeolites maintain the original Brønsted acidity content and high defect content and have virtually no Lewis acidity. They also preserve the “as-made” Al distribution after crystallization and show a clear link between synthesis conditions and divalent cation capacity, as measured with aqueous cobalt ion uptake. The synthesis protocol is found to be the main contributor to Al proximity, yielding record high exchange capacity when ozonated. After conventional calcination at 500–600 °C, however, the presence of water leads to the gradual depletion of Brønsted acid sites, in particular, in small crystals. This work indicates that low-temperature ozonation followed by thermal activation at different temperatures can be used as a novel tool for tuning the amount and nature of acid sites, providing insights into the activity of zeolites in acid-catalyzed reactions, such as CO2 hydrogenation to dimethyl ether, and thereby expanding the possibilities of rational acidity tuning.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


