BCR::ABL1单核苷酸变异对阿西米尼疗效的影响
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
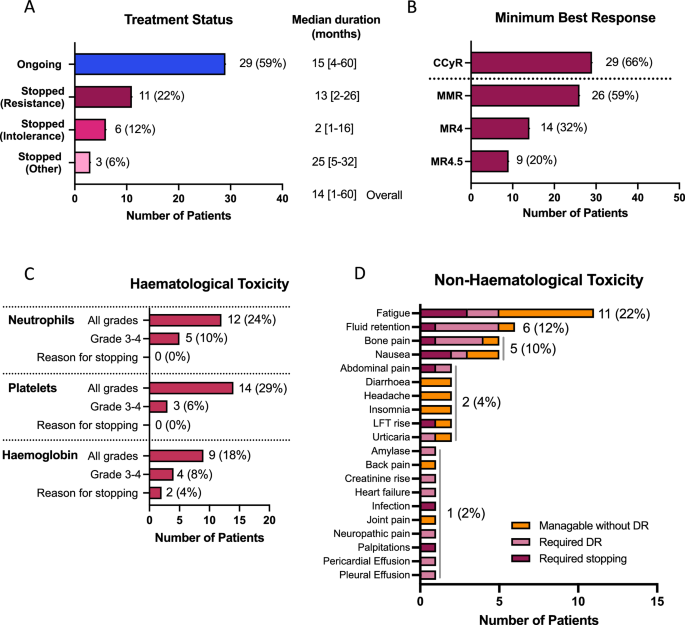
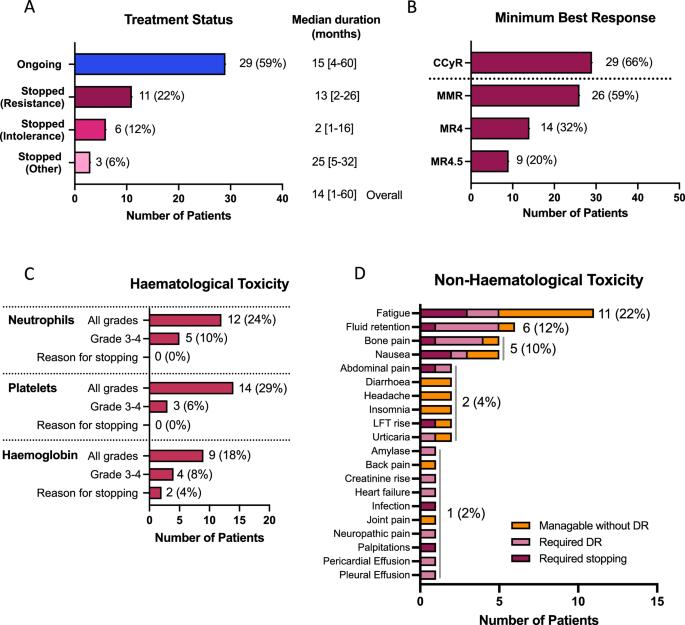
阿西米尼是一种强效的BCR::ABL1选择性抑制剂,具有避免脱靶激酶抑制产生毒性的潜力。在英国,49 名患者在管理准入计划下接受了阿西米尼的治疗,并对其毒性和反应进行了评估。不耐受而非耐药性(65% 对 35%)是停止最后一线治疗的最常见原因,但阿西米尼的耐受性良好,大多数患者(29 人,59%)在中位 14 个月的随访中仍在接受治疗,只有 6 人(12%)因不耐受而停止治疗。在44名可评估反应的患者中,29人(66%)获得了完全细胞遗传学反应(CCyR)或更好的反应,因耐药而停止最后一线治疗的患者反应较差。既往有非T315I-BCR::ABL1单核苷酸变异体(BSNV)病史或基线检测到非T315I-BSNV的患者获得CCyR的人数较少。通过下一代测序对 BSNV 的序列追踪显示,BSNV 邻近群体的克隆扩增在某些情况下与耐药有关(E459K、F317L、F359I),而在另一些情况下则出现在持续应答的背景下,通常与加强剂量有关(T315I、I502F)。这些数据表明,阿西米尼对体内某些 BSNV 相关群体产生了选择性压力,其中一些群体可能会对加强给药产生反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of BCR::ABL1 single nucleotide variants on asciminib efficacy
Asciminib is a potent and selective inhibitor of BCR::ABL1, with potential to avoid toxicity resulting from off-target kinase inhibition. Forty-nine patients treated with asciminib under a managed access program in the UK were evaluated for toxicity and response. Intolerance, rather than resistance (65% vs. 35%), was the most common reason for cessation of the last-line of treatment but asciminib was well tolerated, with most patients (29, 59%) remaining on treatment at a median of 14 months follow-up, and only 6 (12%) stopping for intolerance. Of 44 patients assessable for response, 29 (66%) achieved a complete cytogenetic response (CCyR) or better, with poorer responses seen in those stopping their last-line of therapy for resistance. Fewer patients with a prior history of a non-T315I-BCR::ABL1 single nucleotide variant (BSNV), or a non-T315I-BSNV detectable at baseline achieved CCyR. Serial tracking of BSNV by next generation sequencing demonstrated clonal expansion of BSNV-harbouring populations, which in some settings was associated with resistance (E459K, F317L, F359I), while in others was seen in the context of ongoing response, often with intensified dosing (T315I, I502F). These data suggest that asciminib exerts selective pressure on some BSNV-harbouring populations in vivo, some of which may respond to intensified dosing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


