臭氧铍络合物闭环反应中的重原子隧道效应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
量子力学隧道(QMT)一直被认为是理解化学反应机理的关键,尤其是涉及氢等轻原子的反应。然而,最近的研究结果将这一认识扩展到了重原子隧道反应。在本报告中,我们观察到两个重原子隧道反应,涉及在低温氖基质中,从端对键的臭氧铍复合物 OBeOOO (A) 和 BeOBeOOO (C) 自发转化为相应的侧对键臭氧异构体 OBe(η2-O3) (B) 和 BeOBe(η2-O3) (D)。这一发现得到了速率常数微弱的温度依赖性和异常巨大的 16O/18O 动力同位素效应的支持。量子化学计算显示,这两个闭环反应的壁垒都非常低(1 kcal/mol)。此外,对这两个反应的瞬子理论计算揭示了隧道过程涉及所有四个氧原子的协同运动。本文章由计算机程序翻译,如有差异,请以英文原文为准。
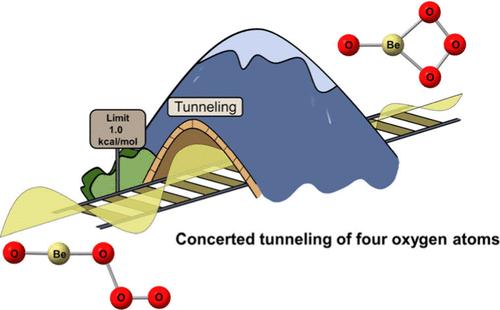
Heavy-Atom Tunneling in Ring-Closure Reactions of Beryllium Ozonide Complexes
Quantum mechanical tunneling (QMT) has long been recognized as crucial for understanding chemical reaction mechanisms, particularly in reactions involving light atoms like hydrogen. However, recent findings have expanded this understanding to include heavy-atom tunneling reactions. In this report, we present the observation of two heavy-atom tunneling reactions involving the spontaneous conversions from end-on bonded beryllium ozonide complexes, OBeOOO (A) and BeOBeOOO (C), to their corresponding side-on bonded ozonide isomers, OBe(η2-O3) (B) and BeOBe(η2-O3) (D), respectively, in a cryogenic neon matrix. This discovery is supported by the weak temperature dependence of the rate constants and unusually large 16O/18O kinetic isotope effects. Quantum chemistry calculations reveal extremely low barriers (<1 kcal/mol) for both ring-closure reactions. Additionally, instanton theory calculations on both reactions unveil that the tunneling processes involve the concerted motion of all four oxygen atoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


