表皮生长因子受体 2(FGFR2)融合确定了胰腺癌中可用于临床的分子亚群
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
少数转移性胰腺导管腺癌(PDAC)中存在成纤维细胞生长因子受体(FGFR)基因的基因组改变,这可能代表了可能从 FGFR 靶向疗法中获益的新兴患者亚群。在这里,我们介绍了四例FGFR2融合阳性的转移性PDAC患者,他们对FGFR激酶抑制剂表现出了持久的反应或疾病控制。利用我们定制的 FGFR 聚焦无细胞 DNA 检测方法 FGFR-Dx,我们在 FGFR 抑制剂治疗期间连续监测了 FGFR2 融合的变异等位基因分数,并观察到了与临床反应相关的动态变化。我们对 30,229 例胰腺癌进行了全面的基因组分析,发现其中 245 例存在 FGFR1-3 融合,发生率为 0.81%。FGFR融合通常与其他已知的致癌基因相互排斥。我们的研究结果为识别FGFR2融合阳性的PDAC患者并用FGFR靶向疗法进行治疗提供了临床证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
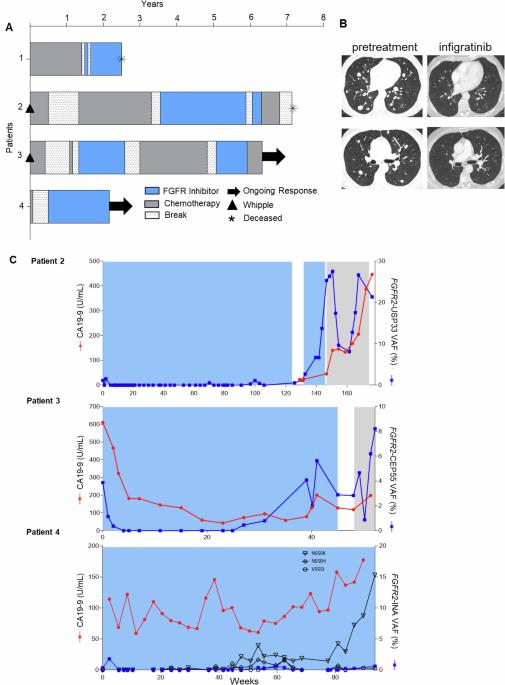
FGFR2-fusions define a clinically actionable molecular subset of pancreatic cancer
Genomic alterations in fibroblast growth factor receptor (FGFR) genes are present in a small number of metastatic pancreatic ductal adenocarcinomas (PDAC) and may represent an emerging subgroup of patients likely to benefit from FGFR targeted therapies. Here we present four FGFR2 fusion-positive metastatic PDAC patients who exhibited durable responses or disease control to FGFR kinase inhibitors. Utilizing our custom FGFR focused cell-free DNA assay, FGFR-Dx, we serially monitored variant allele fractions of FGFR2 fusions during FGFR inhibitor treatment and observed dynamic changes correlating with clinical responses. Genomic analysis of 30,229 comprehensively profiled pancreatic cancers revealed FGFR1-3 fusions in 245 cases, an incidence of 0.81%. FGFR fusions were generally mutually exclusive from other known oncogenes. Our findings provide clinical evidence for identifying and treating FGFR2 fusion-positive PDAC patients with FGFR targeted therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


