沸石-Y 上支持的 Ru 纳米粒子用于苯酚的正苄基化和 H2O2 的活化,以选择性合成 BINOLs† 烷醇
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
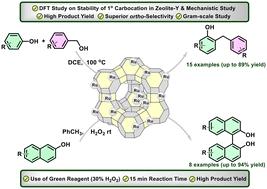
长期以来,酚与芳香族伯醇的苄基化反应以及萘酚与过氧化氢(H2O2)的 C-C 氧化偶联反应一直是有机化学家面临的挑战。本研究利用沸石-NaY 基体支撑的钌 (Ru) 催化剂解决了与这些反应相关的局限性问题。对三种不同类型的 Ru 催化剂进行的详细研究提供了以下信息:在苯酚的苄基化反应中,用高价位 Ru 物创建强 Lewis 酸位点可获得高生产率和高正向选择性。然而,低价 Ru(0) 物种的存在会对催化过程产生负面影响。在 H2O2 的存在下,2-萘酚的 C-C 氧化偶联反应迅速进行,产物仅为 BINOL。使用相同的 Ru 催化剂,苯酚的苄化反应在 100 ℃ 下进行,而萘酚的 C-C 偶联反应在室温下进行得非常顺利。关于苄基碳位的稳定性和反应机理的密度泛函理论(DFT)计算清楚地表明了支撑催化剂的作用及其对催化反应的影响。质谱分析支持的自由基捕获实验表明,2-萘氧基自由基参与了选择性[1,1′-联萘]-2,2′-二醇(BINOL)的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ru-nanoparticles supported on zeolite-Y for ortho-benzylation of phenols and activation of H2O2 for selective synthesis of BINOLs†
Benzylation of phenols with primary aromatic alcohols and the oxidative C–C coupling of naphthols to biaryl derivatives with hydrogen peroxide (H2O2) have long posed challenges for organic chemists. The present work addresses the limitations associated with these reactions using a ruthenium (Ru)-based catalyst supported on a zeolite-NaY matrix. A detailed investigation of three different types of Ru-catalysts provided the information that creation of strong Lewis acid sites with high valent Ru species endorsed high productivity coupled with high ortho-selectivity in the benzylation of phenols. The presence of low-valent Ru(0) species, however, had a negative impact on the catalytic process. The oxidative C–C coupling of 2-naphthol in the presence of H2O2 proceeded rapidly, yielding only BINOL as the product. The benzylation of phenol with the same Ru catalyst occurred at 100 °C while C–C coupling of naphthols proceeded very well at room temperature. Density functional theory (DFT) calculation on the stability of benzyl carbocation and the reaction mechanism clearly highlighted the role of the supported catalyst and its impact on the catalytic reaction. Fee radical trapping experiment supported by mass spectroscopy analysis suggested the involvement of 2-naphthyloxy radicals in selective [1,1′-binaphthalene]-2,2′-diol (BINOL) formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


