用于药物靶标识别的可光释标签--噁二唑啉类
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
光亲和标记是一种广泛用于研究配体-蛋白质和蛋白质-蛋白质相互作用的技术。传统的光亲和标记利用高活性中间体介导的非特异性 C-H 键插入反应。尽管重氮类化合物是应用最广泛的光亲和标签,但它们与下游有机反应的兼容性有限,而且存在储存稳定性问题。本研究将噁二唑啉作为创新的、互补的光活性标签引入工具箱,并通过细胞内标记实验展示了它们在体外的应用。噁二唑啉可以很容易地从酮基合成,并在 302-330 纳米波长的光下裂解,干净利落地释放出重氮活性分子,从而共价修饰亲核氨基酸残基。值得注意的是,噁二唑啉类化合物与各种有机反应条件和官能团兼容,可以探索很大的化学空间。我们制备了几种具有噁二唑啉官能团的已知抑制剂,它们的结合亲和力不受影响。此外,我们还在体外和细胞内证实了噁二唑啉在紫外线照射下与蛋白质形成共价键的能力,其结果与匹配的重氮化合物相当。本文章由计算机程序翻译,如有差异,请以英文原文为准。
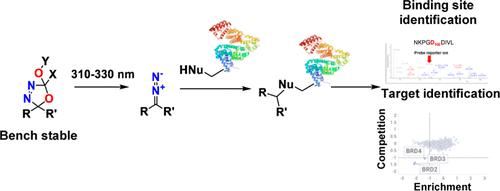
Oxadiazolines as Photoreleasable Labels for Drug Target Identification
Photoaffinity labeling is a widely used technique for studying ligand–protein and protein–protein interactions. Traditional photoaffinity labels utilize nonspecific C–H bond insertion reactions mediated by a highly reactive intermediate. Despite being the most widely used photoaffinity labels, diazirines exhibit limited compatibility with downstream organic reactions and suffer from storage stability concerns. This study introduces oxadiazolines as innovative and complementary photoactivatable labels for addition to the toolbox and demonstrates their application in vitro and through in cellulo labeling experiments. Oxadiazolines can be easily synthesized from ketone moieties and cleaved with 302–330 nm light to cleanly liberate a diazo reactive moiety that can covalently modify nucleophilic amino acid residues. Notably, oxadiazolines are compatible with various organic reaction conditions and functional groups, allowing for the exploration of a large chemical space. Several known inhibitors featuring the oxadiazoline functionality were prepared without affecting their binding affinity. Furthermore, we confirmed the ability of oxadiazolines to form covalent bonds with proteins upon UV-irradiation, both in vitro and in cellulo, yielding comparable results to those of the matched diazirine compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


