促进 1,2-二氯乙烷催化裂解的 B-N 原子对之间的协同作用
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
催化裂解 1,2-二氯乙烷(EDC)以获得氯乙烯(VCM)单体是生产聚氯乙烯(PVC)的关键步骤。掺杂杂原子的碳催化剂表现出理想的性能,但其基本机理仍未完全清楚。本文制备了一系列用于 EDC 催化裂解的 B-N 共掺杂碳 (BNC)、N 掺杂碳 (NC)、B 掺杂碳 (BC) 和纯碳 (C) 催化剂,并仔细研究了 B 和 N 之间的协同作用机理。BNC 催化剂表现出更高的活性,在 250 °C 时的 EDC 转化率高达 53.9%。通过结合实验和理论分析,我们合理地认为,B-N 原子对的形成有助于提高催化剂的性能,B-N 原子对之间的电子相互作用使 N 位点具有更强的碱性,从而将 C-H 键裂解的活化能势垒降低了 0.34 eV。本研究结果为精确设计用于 EDC 裂解的高效非金属碳基催化剂奠定了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
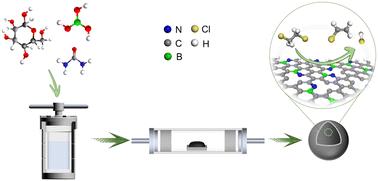
Synergism between B–N atomic pair for promoting the catalytic cracking of 1,2-dichloroethane†
The catalytic cracking of 1,2-dichloroethane (EDC) to obtain vinyl chloride (VCM) monomer is a crucial step in the production of polyvinyl chloride (PVC). The heteroatom-doped carbon catalysts have exhibited desired performance; however, the underlying mechanism is still not fully understood. Herein, a series of B–N co-doped carbon (BNC), N-doped carbon (NC), B-doped carbon (BC) and pure carbon (C) catalysts were prepared for EDC catalytic cracking, and the synergistic mechanism between B and N was carefully investigated. The BNC catalyst exhibits prominently higher activities with an EDC conversion of 53.9% at 250 °C. Through a combination of experimental and theoretical analyses, it is rationalized that the formation of the B–N atomic pair contributes to the enhanced performance and the electronic interaction between the B–N atomic pair imparts greater basicity to the N sites, which reduces the activation energy barrier for C–H bond cleavage by 0.34 eV. The present results provide a theoretical foundation for the precise design of highly efficient non-metallic carbon-based catalysts for EDC cracking.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


