对二甲苯氧化过程中锰催化剂结构和配位的第一原理研究
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
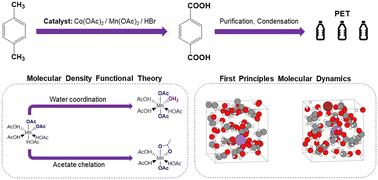
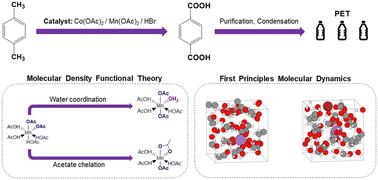
对二甲苯氧化成对苯二甲酸的过程具有全球重要性,其产物可用作聚对苯二甲酸乙二醇酯(PET)的前体。对二甲苯的氧化是通过涉及钴、锰和溴的氧化还原级联进行的,其协同作用可实现高选择性和高反应活性;然而,由于工业操作条件恶劣,催化剂物种的平衡配位环境仍不确定。为了了解催化剂的配位情况并加深对反应过程的理解,本文采用密度泛函理论方法来确定氧化还原级联中二价(还原)和三价(氧化)锰催化剂的静态和动态特性。计算了锰的吉布斯自由能与内配位圈中配体的函数关系,八面体配位的 Mn(OAc)2(HOAc)2 和 Mn(OAc)3(H2O)1 分别被确定为 Mn(II) 和 Mn(III) 热力学上最稳定的配位环境。利用第一原理分子动力学模拟确定了这些催化剂在明确溶剂环境下的动态特性。模拟结果表明,在标准工业温度和压力下,内配位层中存在 0-2 个配位水配体。动力学模拟还扩展到了 HBr,它在氧化还原级联过程中与锰发生偶联,而溴化物没有进入氧化锰(III)催化剂的内配位层,这证明溴化物和锰(III)之间的电子转移是通过外配位层机制进行的。我们的研究结果表明,氧化锰(II)有可能在内层配位环境中促进 L 型配体交换。这些结果为更全面地了解原子尺度的反应机理提供了一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
First principles investigation of manganese catalyst structure and coordination in the p-xylene oxidation process†
The oxidation of p-xylene to terephtalic acid has global importance, with the product used as a precursor for polyethylene terephthalate (PET). The oxidation of p-xylene proceeds via a redox cascade that involves cobalt, manganese, and bromide, with a synergy allowing for high selectivity and reactivity; however, the equilibrium coordination environment of the catalyst species remains uncertain due to the hostile industrial operating conditions. To build knowledge of the catalyst speciation and develop understanding of the reaction process, a density functional theory approach is applied herein to determine the static and dynamic properties of the divalent (reduced) and trivalent (oxidized) manganese catalysts in the redox cascade. The Gibbs free energy has been calculated for manganese as a function of ligands in the inner coordination sphere, with the octahedrally-coordinated Mn(OAc)2(HOAc)2 and Mn(OAc)3(H2O)1 identified as the most thermodynamically stable coordination environments for Mn(ii) and Mn(iii), respectively. Dynamic properties of these catalysts in the presence of an explicit solvent environment have been determined using first principles molecular dynamics simulations. The simulations indicate 0–2 coordinating water ligands are present in the inner coordination sphere under standard industrial temperatures and pressures. The dynamical simulations have been extended to include HBr, which couples with Mn in the redox cascade, and the bromide species does not enter in the inner-coordination sphere of the oxidized Mn(iii) catalyst, providing evidence that the electron transfer between bromide and Mn(iii) proceeds via an outer sphere mechanism. Our results suggest that oxidation of Mn(ii) has the potential for facilitating L-type ligand exchange in the inner-sphere coordination environment. The results are a platform for developing a more complete knowledge of the reaction mechanism at the atomistic scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


