喹啉基取代的硫代碳酸酰胺分子内杂环化为功能化的 2,4- 二氢-3H-1,2,4-三唑和-1,3,4-噻二唑
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
和 3-[2-(5-苯胺基-1,3,4-噻二唑-2-基)乙基] 4-甲基喹啉-2(1H)-酮。这些反应经济高效,在温和条件下生成的目标产物收率高(85-98%),无需使用昂贵的催化剂和柱层析分离。本文章由计算机程序翻译,如有差异,请以英文原文为准。
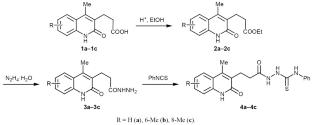
Intramolecular Heterocyclization of Quinolyl-Substituted Carbothioamides to Functionalized 2,4-Dihydro-3H-1,2,4-triazoles and -1,3,4-thiadiazoles
A simple and economical approach has been proposed for the transformation of 2-(quinolylpropanoyl)-N-phenylhydrazine-1-carbothioamide into 4-methyl-3-[2-(4-phenyl-5-sulfanylidene-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethyl]quinolin-2(1H)-ones and 3-[2-(5-anilino-1,3,4-thiadiazol-2-yl)ethyl] 4-methylquinolin-2(1H)-ones by heterocyclization in the presence of aqueous sodium hydroxide and concentrated sulfuric acid, respectively. The reactions are economically efficient, and the target products are formed in high yields (85–98%) under mild conditions without using expensive catalysts and column chromatography for their isolation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


