生物基质中特殊促溶解脂质介质和经典二十酸的鉴定方法的开发与验证
IF 3.1
2区 化学
Q2 BIOCHEMICAL RESEARCH METHODS
Journal of the American Society for Mass Spectrometry
Pub Date : 2024-09-09
DOI:10.1021/jasms.4c00211
引用次数: 0
摘要
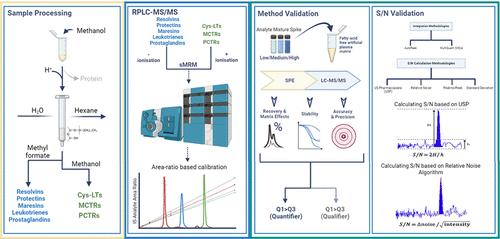
脂质介质包括专门的促消炎介质和传统的类二十烷酸,在引发和消除炎症方面都起着关键作用。这些分子的调节决定了炎症是自然消退还是持续存在。然而,我们对这些介质在各种炎症环境中如何随着时间的推移而被调节的了解还很有限。这一空白阻碍了我们对疾病发生和发展机制的了解。由于这些介质在许多组织中具有局部作用且内源性水平较低,因此必须开发稳健、高灵敏度的方法来评估它们在各种炎症环境中的内源性调控。这些方法将有助于我们深入了解它们的生理作用。在这里,我们建立了提取、鉴定和量化这些介质的方法。利用我们的方法,我们共鉴定出 37 种脂质介质。此外,通过采用反相高效液相色谱法,我们成功分离了部分脂质介质的双键异构体和手性异构体,包括脂氧素(LX)A4、15-epi-LXA4、保护素(PD)D1、PDX 和 17R-PD1。在溶剂和替代基质中对该方法的标准曲线线性、定量下限(LLOQ)、准确度和精密度进行了验证。研究结果表明,该方法线性关系良好,r2 值为 0.98,在相中介质的定量下限为 0.01 至 0.9 pg,在代用基质中介质的定量下限为 0.1 至 8.5 pg。在溶剂中,低、中、高浓度下,日间和日内精密度的相对标准偏差(RSD)为 5%至 12%,而在低浓度至高浓度下,准确度的日间和日内变异性 RSD 为 95%至 87%。所用内标在生物基质(血浆和血清)中的回收率为 60% 至 118%。我们观察到在负离子模式下评估的分子存在明显的离子抑制现象,而在正离子模式下评估的分子则存在基质离子增强效应。对自动峰值和 MQ4 等积分算法以及信噪比计算方法(即美国药典、相对噪音、峰对峰和标准偏差)进行比较后发现,所测试的不同积分算法对信噪比计算的影响很小。相比之下,用于计算信噪比的方法对结果的影响更大,而相对噪声方法被证明是最稳健的。本文介绍的方法为研究生物组织中的 SPM 和经典类二十酸提供了一个平台,有助于我们进一步了解疾病的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development and Validation of Methodologies for the Identification of Specialized Pro-Resolving Lipid Mediators and Classic Eicosanoids in Biological Matrices
Lipid mediators, which include specialized pro-resolving mediators and classic eicosanoids, are pivotal in both initiating and resolving inflammation. The regulation of these molecules determines whether inflammation resolves naturally or persists. However, our understanding of how these mediators are regulated over time in various inflammatory contexts is limited. This gap hinders our grasp of the mechanisms underlying the disease onset and progression. Due to their localized action and low endogenous levels in many tissues, developing robust and highly sensitive methodologies is imperative for assessing their endogenous regulation in diverse inflammatory settings. These methodologies will help us gain insight into their physiological roles. Here, we establish methodologies for extracting, identifying, and quantifying these mediators. Using our methods, we identified a total of 37 lipid mediators. Additionally, by employing a reverse-phase HPLC method, we successfully separated both double-bond and chiral isomers of select lipid mediators, including Lipoxin (LX) A4, 15-epi-LXA4, Protectin (PD) D1, PDX, and 17R-PD1. Validation of the method was performed in both solvent and surrogate matrix for linearity of the standard curves, lower limits of quantitation (LLOQ), accuracy, and precision. Results from these studies demonstrated that linearity was good with r2 values > 0.98, and LLOQ for the mediators ranged from 0.01 to 0.9 pg in phase and from 0.1 to 8.5 pg in surrogate matrix. The relative standard deviation (RSD) for inter- and intraday precision in solvent ranged from 5% to 12% at low, intermediate, and high concentrations, whereas the RSD for the inter- and intraday variability in the accuracy ranged from 95% to 87% at low to high concentrations. The recovery in biological matrices (plasma and serum) for the internal standards used ranged from 60% to 118%. We observed a marked ion suppression for molecules evaluated in negative ionization mode, while there was an ion enhancement effect by the matrix for molecules evaluated in positive ionization mode. Comparison of the integration algorithms, namely, AutoPeak and MQ4, and approaches for calculating signal-to-noise ratios (i.e., US Pharmacopeia, relative noise, peak to peak, and standard deviation) demonstrated that different integration algorithms tested had little influence on signal-to-noise ratio calculations. In contrast, the method used to calculate the signal-to-noise ratio had a more significant effect on the results, with the relative noise approach proving to be the most robust. The methods described herein provide a platform to study the SPM and classic eicosanoids in biological tissues that will help further our understanding of disease mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.50
自引率
9.40%
发文量
257
审稿时长
1 months
期刊介绍:
The Journal of the American Society for Mass Spectrometry presents research papers covering all aspects of mass spectrometry, incorporating coverage of fields of scientific inquiry in which mass spectrometry can play a role.
Comprehensive in scope, the journal publishes papers on both fundamentals and applications of mass spectrometry. Fundamental subjects include instrumentation principles, design, and demonstration, structures and chemical properties of gas-phase ions, studies of thermodynamic properties, ion spectroscopy, chemical kinetics, mechanisms of ionization, theories of ion fragmentation, cluster ions, and potential energy surfaces. In addition to full papers, the journal offers Communications, Application Notes, and Accounts and Perspectives

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


