三氟化铁和 γ-环糊精催化烯烃与 1-萘酚和 2-萘酚的加氢反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
展示了一种由 Fe(OTf)3 和 γ-环糊精催化的 1-萘酚或 2-萘酚与烯烃的加氢反应。这种高效而通用的方法可从易于获得的起始材料中得到多种苄基萘酚,具有很高的化学和区域选择性,收率高达 99%,且无需强碱或添加剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
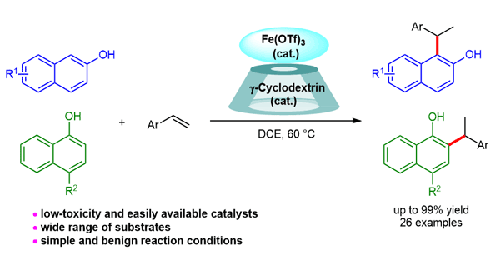
Iron(III) Triflate and γ-Cyclodextrin-Catalyzed Hydroarylation of Alkenes with 1-Naphthols and 2-Naphthols
A Fe(OTf)3 and γ-cyclodextrin-catalyzed hydroarylation of alkenes with 1-naphthols or 2-naphthols is demonstrated. This efficient and general method delivers a wide range of benzylic naphthols from readily available starting materials with high chemo- and regioselectivity in up to 99% yield, with no need for a strong base or additive.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


