用手性次碘酸双胍催化剂对 2-氧化吲哚进行对映选择性氧化偶联反应
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
双吲哚基团存在于多种具有生物活性的化合物中。在此,我们报告了在手性双胍基次碘酸催化剂存在下,2-氧化吲哚的对映体选择性氧化同偶联反应,以优异的产率和中等至高的非对映及对映体选择性获得了相应的光学活性双氧化吲哚。本文章由计算机程序翻译,如有差异,请以英文原文为准。
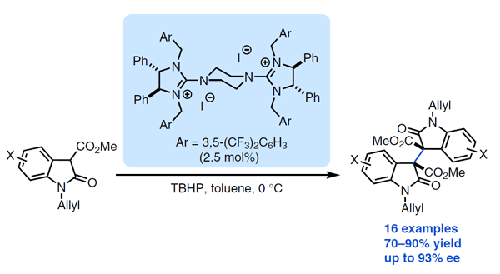
Enantioselective Oxidative Homocoupling of 2-Oxindoles with a Chiral Bisguanidinium Hypoiodite Catalyst
The bisoxindole motif is present in a variety of biologically active compounds. Here, we report an enantioselective oxidative homocoupling reaction of 2-oxindoles in the presence of a chiral bisguanidinium hypoiodite catalyst, providing access to the corresponding optically active bisoxindoles in excellent yields and with moderate to high diastereo- and enantioselectivities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


