含有海因和咪唑烷酮分子的两种奎奎宁酸类似物的改进合成方法
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
鉴于最近在开发 SARS-CoV-2 主要蛋白酶抑制剂方面取得的进展,其关键片段杂环氨基酸的合成备受关注。在此,我们报告了一种制备含有海因和咪唑烷酮分子的两种新奎喹酸类似物的方法。海因类似物是通过酰胺酯环化反应获得的,而咪唑烷酮单元则是通过还原胺化反应以及随后的取代乙二胺与羰基二咪唑环化反应生成的。所介绍的方法分别只需 8 个和 5 个步骤就能聚合合成目标类似物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
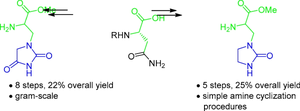
Improved synthesis of two quisqualic acid analogs containing hydantoin and imidazolidinone moieties
In the light of recent progress in the development of SARS-CoV-2 main protease inhibitors, the synthesis of their key fragment, heterocyclic amino acids, is of great interest. Here, we report a method for the preparation of two new quisqualic acid analogs containing hydantoin and imidazolidinone moieties. The hydantoin analog was obtained using an amide ester cyclization, while the imidazolidinone unit was constructed by reductive amination and subsequent cyclization of a substituted ethylenediamine with carbonyldiimidazole. The presented approach provides the convergent synthesis of target analogs in 8 and 5 steps respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


