ZG16 通过诱导 CD40 促进树突状细胞的成熟,有助于胰腺癌的抗肿瘤免疫
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
树突状细胞(DC)是抗原诱导和 T 细胞活化的关键介质。在T细胞介导的抗肿瘤免疫中,嗜酸性粒细胞蛋白16(ZG16)被证明是一种抗癌基因,但它对DC的影响在很大程度上是未知的。在此,我们想知道ZG16是否会影响胰腺癌中DC的活化。首先,我们观察到 ZG16 在小鼠骨髓或人类外周血 DCs 成熟过程中表达增加。然后,过表达ZG16或外源引入重组ZG16蛋白可诱导DC表面MHC II、CD86、CD84和CCR7的表达,从而促进促炎介质IL-1β、IL-6、TNF-α和IL-12/p70的分泌,支持ZG16对DC成熟的促进作用。通过建立胰腺癌小鼠皮下和正位模型,我们证实腹腔注射重组ZG16蛋白(Re-mZG16)可通过刺激DC成熟和增强CD4 +、CD8 +、PD-1 +和Ctla4+细胞的抗肿瘤反应诱导肿瘤消退。此外,Re-mZG16 与吉西他滨联合治疗胰腺癌具有协同作用。从机理上讲,我们证明了ZG16能抑制CD40的泛素化和降解,而这取决于ZG16的凝集素结构域。总之,这项研究为ZG16-CD40轴在基于DC的胰腺癌免疫疗法中的作用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
ZG16 enhances the maturation of dendritic cells via induction of CD40 and contributes to the antitumor immunity in pancreatic cancer
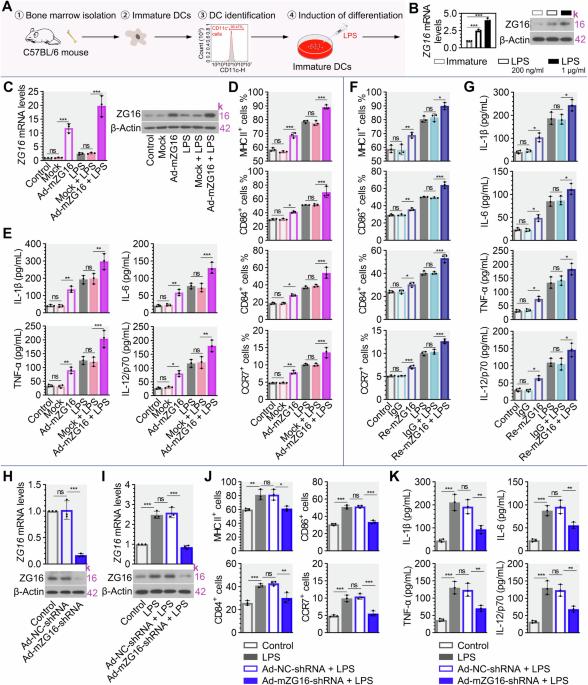
Dendritic cells (DCs) are critical mediators of antigen priming and T-cell activation. Zymogen granule protein 16 (ZG16) is demonstrated as an anti-oncogene in T-cell mediated antitumor immunity, but its effect on DCs is largely unknown. Herein, we wonder whether ZG16 affects the activation of DCs in pancreatic cancer. Firstly, the increased ZG16 expression was observed during the maturation of DCs derived from mouse bone marrow or human peripheral blood. Then, overexpression of ZG16 or exogenous introduction of recombinant ZG16 protein induced the expression of MHC II, CD86, CD84, and CCR7 on the surface of DCs, thereby facilitating the secretion of proinflammatory mediators IL-1β, IL-6, TNF-α, and IL-12/p70, supporting the promoting effect of ZG16 on DC maturation. By establishing the subcutaneous and orthotopic mouse models of pancreatic cancer, we confirmed that intraperitoneal injection of recombinant ZG16 protein (Re-mZG16) could induce tumor regression by stimulating DC maturation and enhancing antitumor responses of CD4 + , CD8 + , PD-1 + , and Ctla4+ cells. Besides, Re-mZG16 in combination with gemcitabine showed a synergistic effect in the treatment of pancreatic cancer. Mechanistically, we demonstrated that ZG16 inhibited the ubiquitination and degradation of CD40, which depended on the lectin domain of ZG16. In conclusion, this study provided a novel insight into the role of ZG16-CD40 axis in DC-based immunotherapy for pancreatic cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


