Nb2O5-supported 氧化钴在催化甲苯氧化中的形态依赖性
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
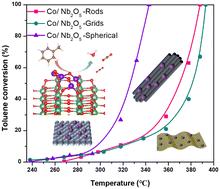
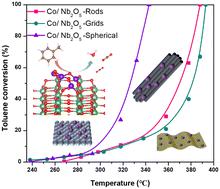
本研究介绍了以不同形式的 Nb2O5(Nb2O5-R)、网格(Nb2O5-G)和球形结构(Nb2O5-S)为载体的钴基催化剂的制备过程。这些催化剂在甲苯氧化过程中表现出不同的反应活性,这与它们各自的物理和化学特性以及氧化钴和 Nb2O5 载体之间的界面相互作用有关。值得注意的是,球形 Nb2O5 载体(CoOx/Nb2O5-S)催化剂的性能优于其他载体的催化剂,在氧化甲苯方面表现出最佳活性。这项研究强调了 Nb2O5 衬底的独特特性在提高催化剂吸附和活化甲苯的功效方面所起的作用。密度泛函理论(DFT)显示,CoOx/Nb2O5-S 更容易吸附甲苯(-0.65 eV),氧空位的产生和吸附所需的能量也更少。这表明 CoOx/Nb2O5-S 催化剂增强了表面氧的流动性,提高了催化效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Morphology dependence of Nb2O5-supported cobalt oxide in catalytic toluene oxidation†
This research describes the preparation of cobalt-based catalysts supported on Nb2O5 substrates of various forms: rods (Nb2O5-R), grids (Nb2O5-G), and spherical structures (Nb2O5-S). These catalysts demonstrated diverse reactivity in toluene oxidation, which correlated with their individual physical and chemical traits and the interfacial interaction between cobalt oxide and the Nb2O5 support. Notably, the catalyst with a spherical Nb2O5 support (CoOx/Nb2O5-S) outperformed the catalysts with other supports and showed the best activity in oxidizing toluene. The investigation underscored the role of the unique features of the Nb2O5 substrate in augmenting the catalyst's efficacy in toluene adsorption and activation. Density functional theory (DFT) revealed more facile toluene adsorption on CoOx/Nb2O5-S (−0.65 eV) and reduced energy requirements for oxygen vacancy creation and adsorption. This suggested that the CoOx/Nb2O5-S catalyst enhanced surface oxygen mobility and boosted catalytic efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


