碳支撑的 Zn-HPW 配体乙炔水合催化剂
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
近年来,利用丰富的原料生产乙醛仍具有很高的研究价值。为了解决锌基催化剂在反应过程中活性组分易流失和积碳的问题,研究人员制备了一系列 Zn-O4 构型的 Zn-HPW/AC 催化剂。表征结果表明,磷钨酸(HPW)配体有效地促进了 Zn 物种的分散,提供了更多的酸性位点,减轻了 Zn 的损失,提高了催化剂的抗积碳性能。密度泛函理论(DFT)计算进一步证实,水分子优先吸附在催化剂表面,促进水分子离解,水分子与 Zn 离解的 H 形成最稳定的 Zn-OH 构型,是反应的主要活性中心。同时,水分子离解出的 -OH 与 C2H2 加成,H 还原催化剂,配体催化剂中原有的 H 原子进一步参与反应,实现催化循环。这为乙炔水合绿色催化剂的开发提供了新思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
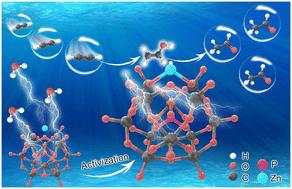
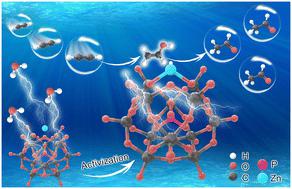
Carbon-supported Zn-HPW ligand catalysts for acetylene hydration†
In recent years, the production of acetaldehyde with rich raw materials still has high research value. A series of Zn-HPW/AC catalysts with a Zn–O4 configuration were prepared to solve the problems of easy loss of active components and carbon accumulation of Zn-based catalysts in the reaction. The characterization results showed that the phosphotungstic acid (HPW) ligands effectively promoted Zn species dispersion, provided more acid sites, mitigated the loss of Zn, and improved the carbon deposition resistance of the catalyst. The density functional theory (DFT) calculation further confirmed that the water molecules preferentially adsorb on the surface of the catalyst to promote the dissociation of water molecules, and the H of dissociation from water molecules and Zn forms the most stable Zn–OH configuration, which is the main active center of the reaction. Meanwhile the –OH dissociated from water molecules is adducted with C2H2, while H reduces the catalyst, and the original H atoms in the ligand catalyst further participate in the reaction to realize the catalytic cycle. This provides a new idea for the development of green catalysts for acetylene hydration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


