通过氢化物转移实现氢溢出:氧化锌和二氧化锆与强氢化物供体的反应
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氢溢出是指 H2 从金属表面转移到支持物(通常是金属氧化物)上,这对许多异相催化过程(包括 Cu/ZnO 和 Cu/ZrO2 催化的甲醇合成)至关重要。由于金属-金属氧化物界面的高度复杂性,人们对 ZnO 或 ZrO2 上的氢溢出知之甚少。在这里,我们通过让 ZnO 和 ZrO2 与分子金属氢化物反应来模拟氢在它们上的溢出,从而了解氢化物的性质如何影响氢的溢出。虽然良好的氢供体 HV(CO)4dppe (1) 和 CpCr(CO)3H (2) 不会与金属氧化物表面发生反应,但强氢化物供体 iBu2AlH (3)、Cp2ZrHCl (4) 和 [HCu(PPh3)]6 (5) 却会还原 ZnO 和 ZrO2,从而产生与通过氢溢出获得的 EPR 信号相同的缺陷位点。我们还利用 X 射线吸收光谱(XAS)观察到表面出现了新的 M-O 键。我们提出,这些金属氧化物通过最初的氢化物转移进行氢溢出,然后表面氢化物发生同分异构,产生还原位点和 OH 键。这种机制与传统的涉及质子和电子转移步骤的溢出机制不同。我们还观察到 ZnO 比 ZrO2 更容易还原,这也解释了为什么在 Cu/ZrO2 上很难观察到溢出现象。本文章由计算机程序翻译,如有差异,请以英文原文为准。
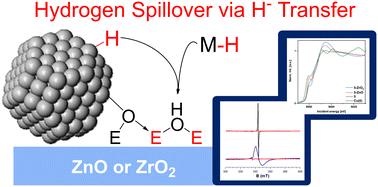
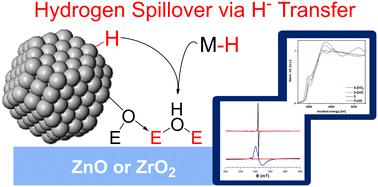
Hydrogen spillover through hydride transfer: the reaction of ZnO and ZrO2 with strong hydride donors†
Hydrogen spillover, transfer of H2 from a metal surface to a support (often metal oxides), is pivotal for many heterogeneous catalytic processes, including Cu/ZnO and Cu/ZrO2 catalyzed methanol synthesis. Little is known about hydrogen spillover on ZnO or ZrO2, due to the high complexity of the metal–metal oxide interface. Here, we model hydrogen spillover on ZnO and ZrO2 by reacting them with molecular metal hydrides to see how the properties of the hydrides affect hydrogen spillover. While the good H· donors HV(CO)4dppe (1) and CpCr(CO)3H (2) do not react with the metal oxide surfaces, the strong hydride donors iBu2AlH (3), Cp2ZrHCl (4), and [HCu(PPh3)]6 (5) do reduce ZnO and ZrO2 to give defect sites with the same EPR signatures as obtained via hydrogen spillover. We also observe new M–O bonds to the surface using X-ray absorption spectroscopy (XAS). We propose that these metal oxides undergo hydrogen spillover via initial hydride transfer followed by tautomerization of the surface hydride, giving reduced sites and OH bonds. This mechanism is in contrast to the traditional spillover mechanism involving discrete proton- and electron transfer steps. We also observe that ZnO is easier to reduce than ZrO2, explaining the difficulty observing spillover on Cu/ZrO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


