利用形状工程 MnOx 纳米催化剂将废旧 PET 瓶高效乙二醇化为高质量的有价单体
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
废旧聚对苯二甲酸乙二醇酯(PET)瓶是现代社会广泛使用的一种塑料,对其进行化学回收利用以获得有价值的单体,为解决消费后塑料相关的环境问题提供了一种前景广阔的解决方案。在这项研究中,我们开发了一种高效的异相催化方法,利用具有明确棒状形态的形状工程化氧化锰(MnOx)纳米催化剂,在温和的条件下促进 PET 与生物质衍生乙二醇的乙二醇化,从而生产出高质量的对苯二甲酸二(2-羟乙基)酯(BHET)有价单体。氧化锰材料的纳米棒形态,特别是在 500 °C 煅烧的氧化锰(MnOx-500),在将废旧 PET 瓶转化为 BHET 的过程中表现出显著的催化效率。在 180 °C 的温度下煅烧 3 小时后,MnOx-500 纳米催化剂实现了 PET 的完全转化,分离出的 BHET 产率达到 86%,超过了 CeO2、TiO2 和 Nb2O5 等各种金属氧化物的性能。利用 NMR、FT-IR、HR-MS 和粉末 XRD 对分离出的 BHET 单体晶体进行了定性分析,并通过 TGA 和 DSC 研究对热稳定性进行了评估。此外,该研究还证明了催化剂的稳定性和可重复使用性,表明这种方法具有实际应用潜力。通过对纳米结构 MnOx 材料进行综合表征,发现了结构与活性之间的相关性,突出了 MnOx-500 纳米催化剂中的氧空位缺陷和酸性特性在高效 PET 糖解以获得所需的 BHET 单体中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
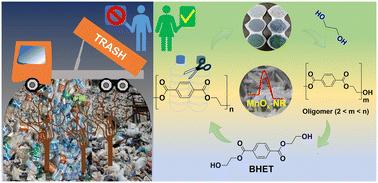
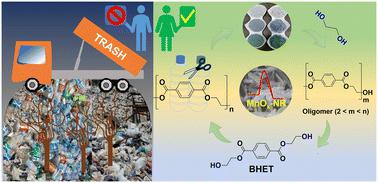
Efficient glycolysis of used PET bottles into a high-quality valuable monomer using a shape-engineered MnOx nanocatalyst†
The chemical recycling of used polyethylene terephthalate (PET) bottles, a widely used plastic in the modern world, to obtain valuable monomers offers a promising solution to address post-consumer plastic-related environmental concerns. In this study, we have developed an efficient heterogeneous catalytic approach using a shape-engineered manganese oxide (MnOx) nanocatalyst with a well-defined rod morphology to facilitate the glycolysis of PET with biomass-derived ethylene glycol to produce a high-quality bis(2-hydroxyethyl) terephthalate (BHET) valuable monomer under mild conditions. The nanorod morphology of the MnOx material, specifically the MnOx calcined at 500 °C (MnOx-500), exhibited remarkable catalytic efficiency in converting used PET bottles into BHET. At a temperature of 180 °C for 3 h, the MnOx-500 nanocatalyst achieved a complete conversion of PET with a 86% isolated yield of BHET, surpassing the performance of various metal oxides, such as CeO2, TiO2, and Nb2O5. Qualitative analysis of the isolated BHET monomer crystals was conducted using NMR, FT-IR, HR-MS, and powder XRD, along with assessments of thermal stability through TGA and DSC studies. Furthermore, the study demonstrated the catalyst's stability and reusability, suggesting the practical application potential of this methodology. The structure–activity correlation, revealed through comprehensive characterization of the nanostructured MnOx materials, highlighted the crucial role of the oxygen vacancy defects and the acidic properties in the MnOx-500 nanocatalyst for efficient PET glycolysis to obtain the desired BHET monomer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


