用于联合生产氢气和增值甲酸盐的选择性甲醇氧化电催化剂的最新进展
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
阳极氧进化反应(OER)的动力学缓慢且过电位大,严重阻碍了传统的水分离过程。因此,用热力学上更有利的有机物氧化反应取代阳极氧进化反应,并与氢进化反应(HER)相结合,是一种获得绿色氢气的创新策略。在这种情况下,甲醇的电重整与电化学 HER 相结合,可实现甲酸盐和氢气的节能共生增值。因此,控制甲醇氧化过程并使其有选择地转化为甲酸盐成为一个值得研究的课题。迄今为止,针对选择性甲醇氧化反应(SMOR)已开发出多种催化剂和改性策略。过渡金属基材料是研究最多的催化剂,因为它们的催化能力适中,能更好地控制甲醇氧化过程。电子结构调控是提高催化剂 SMOR 性能的最有效策略。然而,有关 SMOR 的系统综述报道却很少。鉴于最近取得的重大进展,我们在此综述了用于联合生产增值甲酸酯和绿色氢气的 SMOR 电催化剂的最新进展。其中,首先介绍了 SMOR 的机理,包括传统的表面吸附机理和新开发的晶格氧参与机理。随后,从化学键激活/抑制、电子结构操作、双活性位点构建和增加活性位点数量等方面分析了催化剂设计策略。随后,讨论了涉及电化学测量和产物检测的性能描述指标,以显示基本的评价标准,并根据催化剂的组成对各种 SMOR 催化剂进行分类,以显示催化剂的发展情况。最后,提出了结论和展望。我们希望这些全面的努力将有助于 SMOR 的文献调查,并为 SMOR 研究界提供启发,吸引更多的人关注有机物的电升压与绿色制氢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
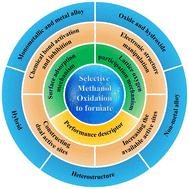
Recent advances in selective methanol oxidation electrocatalysts for the co-production of hydrogen and value-added formate†
Traditional water splitting is significantly impeded by the sluggish kinetics and large overpotential of the anodic oxygen evolution reaction (OER). Accordingly, replacing the OER with a more thermodynamically favorable organic substance oxidation reaction to combine with the hydrogen evolution reaction (HER) is an innovative strategy to obtain green hydrogen. In this case, the electro-reforming of methanol coupled with the electrochemical HER can realize the energy-saving co-generation of value-added formate and hydrogen. Therefore, controlling the process of methanol oxidation and making it selectively transform to formate have become a worthy topic. Thus far, various catalysts and modification strategies have been developed for the selective methanol oxidation reaction (SMOR). Transition metal-based materials are the most studied catalysts because their moderate catalytic ability can better control the process of methanol oxidation. Electronic structure modulation is the most efficient strategy to improve the SMOR performance of catalysts. However, few systematic reviews on the SMOR have been reported. In light of significant advances achieved recently, herein, we reviewed the recent advances in SMOR electrocatalysts for the co-production of value-added formate and green hydrogen. In particular, the mechanism of the SMOR is initially introduced, including the traditional surface adsorption mechanism and the newly developed lattice oxygen participation mechanism. Subsequently, strategies for catalyst design are analyzed from the aspects of chemical bond activation/inhibition, electronic structure manipulation, dual active site construction, and increasing the number of active sites. Thereafter, performance descriptors involving electrochemical measurements and product detection are discussed to show the basic evaluation criterion, and various catalysts for the SMOR are categorized according to their composition to display the development of catalysts. Finally, conclusions and perspectives are presented. We hope that this comprehensive effort will be helpful in the literature survey of the SMOR and provide inspiration to the SMOR research community, attracting more attention to the electro-upgradation of organic substances coupled with green hydrogen generation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


