Pt-CeO2 催化剂在丙烷氧化过程中不同初始尺寸铂的演变
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
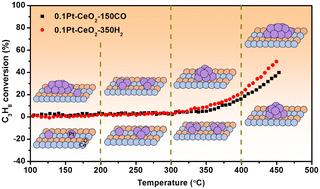
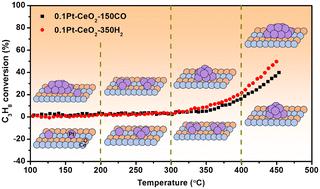
铂基催化剂广泛应用于碳氢化合物的催化燃烧,在排放控制方面发挥着重要作用。然而,开发在低温条件下高效转化碳氢化合物的铂催化剂仍具有挑战性。本文研究了 Pt-CeO2 催化剂上铂尺寸与 C3H8 氧化活性之间的结构性能关系。通过不同的还原处理,得到了单原子和纳米颗粒等不同铂初始状态的样品。两种催化剂在 C3H8 氧化性能上没有明显差异。在 C3H8 氧化过程中,铂的状态是动态变化的,其演变行为与铂的初始状态密切相关;铂单原子不断烧结成团簇,然后随着反应温度的升高转变成铂纳米颗粒,而初始铂纳米颗粒先分散成小团簇,然后再重新烧结成纳米颗粒。根据 DFT 分析得出的结论是,C3H8 和 O2 在不同尺寸的铂粒子上的不同吸附特性导致了它们在 C3H8 氧化过程中的不同演化行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The evolution of Pt with different initial sizes during propane oxidation over Pt–CeO2 catalysts†
Pt-based catalysts are widely used in catalytic combustion of hydrocarbons and play an important role in emission control. However, developing a Pt catalyst for efficient conversion of hydrocarbons at low temperatures remains challenging. Herein, the structure–performance relationship between Pt size and C3H8 oxidation activity was studied over Pt–CeO2 catalysts. The samples with different Pt initial states of single atoms and nanoparticles were obtained by different reduction treatments. No obvious differences were found between the two catalysts in the performances of C3H8 oxidation. The Pt states were found to be dynamically changing during C3H8 oxidation, and the evolution behaviors were closely related to the Pt initial states; Pt single atoms continuously sintered into clusters and then transformed into Pt nanoparticles with elevated reaction temperatures, while initial Pt nanoparticles firstly dispersed into small clusters and then re-sintered into nanoparticles. It is concluded that the different adsorption properties of C3H8 and O2 on Pt species with different sizes are responsible for their different evolution behaviors during C3H8 oxidation based on DFT analyses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


