研究科波维酮特性和热熔挤压工艺对无定形固体分散体产品的杂质含量、体外释放和稳定性的影响
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
热熔挤出法(HME)是一种广泛用于制造难溶性药物的无定形固体分散体(ASD)的方法,在这种方法中,药物分子分散在固体聚合物基质中。本研究探讨了三种不同的 copovidone 辅料、其活性杂质含量、HME 桶温度以及胶体二氧化硅(SiO2)的分布对 ASD 及其片剂的杂质含量、稳定性和药物释放的影响。与 Plasdone S630 Ultra(PS630U)相比,Kollidon VA 64(KVA64)和 Plasdone S630(PS630)的初始过氧化物含量更高,导致新鲜 ASD 片剂中药物的氧化降解程度更高。然而,稳定性测试(50 °C,密闭容器,50 °C/30%相对湿度,开放条件)显示,在较高的桶温下制备的 ASD 片剂中氧化降解杂质较少,这可能是由于过氧化物降解较多所致。Plasdone S630 适用于含有易氧化降解药物的 ASD,而标准纯度等级可能有利于易受自由基降解的药物,因为它们在 HME 后产生的自由基较少。ASD 药片比研磨挤出样品具有更高的物理稳定性,这可能是由于在片剂基质中暴露于稳定性条件的时间减少了。在挤出物成分中加入二氧化硅可提高片剂中 ASD 系统的物理稳定性,但会对化学稳定性产生负面影响,促进药物的氧化降解和羟基化。二氧化硅的分布对药物释放没有影响。该研究还利用流动核磁共振和在线紫外/可见光谱证实了共聚维酮、药物和吐温 80 的一致释放。这项研究强调了过氧化物水平和二氧化硅在影响 ASD 的溶解和物理化学稳定性方面的关键作用。研究结果为开发稳定有效的药物制剂提供了宝贵的见解,强调了在使用 HME 制备的 ASD 产品中控制活性杂质和辅料特性的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
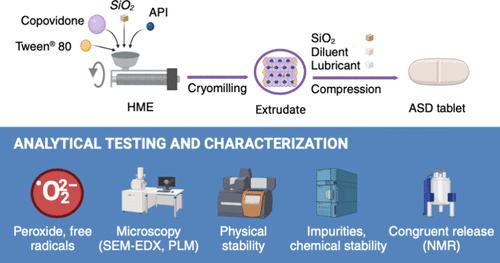
Investigation of the Influence of Copovidone Properties and Hot-Melt Extrusion Process on Level of Impurities, In Vitro Release, and Stability of an Amorphous Solid Dispersion Product
Hot-melt extrusion (HME) is a widely used method for creating amorphous solid dispersions (ASDs) of poorly soluble drug substances, where the drug is molecularly dispersed in a solid polymer matrix. This study examines the impact of three different copovidone excipients, their reactive impurity levels, HME barrel temperature, and the distribution of colloidal silicon dioxide (SiO2) on impurity levels, stability, and drug release of ASDs and their tablets. Initial peroxide levels were higher in Kollidon VA 64 (KVA64) and Plasdone S630 (PS630) compared to Plasdone S630 Ultra (PS630U), leading to greater oxidative degradation of the drug in fresh ASD tablets. However, stability testing (50 °C, closed container, 50 °C/30% RH, open conditions) showed lower oxidative degradation impurities in ASD tablets prepared at higher barrel temperatures, likely due to greater peroxide degradation. Plasdone S630 is suitable for ASDs with drugs prone to oxidative degradation, while standard purity grades may benefit drugs susceptible to free radical degradation, as they generate fewer free radicals post-HME. ASD tablets exhibited greater physical stability than milled extrudate samples, likely due to reduced exposure to stability conditions within the tablet matrix. Including SiO2 in the extrudate composition resulted in greater physical stability of the ASD system in the tablet; however, it negatively affected chemical stability, promoting greater oxidative degradation and hydroxylation of the drug substance. No impact of the distribution of SiO2 on drug release was observed. The study also confirmed the congruent release of copovidone, the drug substance, and Tween 80 using flow NMR coupled with in-line UV/vis. This research highlights the critical roles of peroxide levels and SiO2 in influencing the dissolution and physical and chemical stability of ASDs. The findings provide valuable insights for developing stable and effective pharmaceutical formulations, emphasizing the importance of controlling reactive impurities and excipient characteristics in ASD products prepared by using HME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


