研究抗体与阿尔法-突触核蛋白结合的机制以治疗帕金森病
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
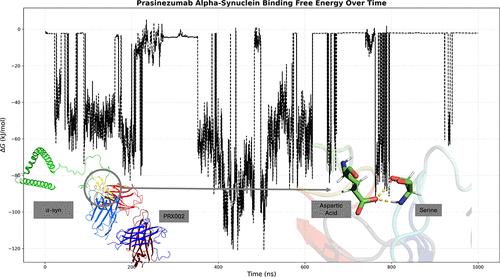
帕金森病(PD)是一种特发性神经退行性疾病,发病率仅次于阿尔茨海默病。帕金森病的病理生理学特征是黑质部位的多巴胺能神经元发生变性,并含有被称为路易体的α-突触核蛋白(α-syn)错误折叠聚集体。尽管对潜在的帕金森病治疗方法进行了数十年的研究,但至今尚未开发出任何一种治疗方法,而开发新的治疗药物是一个耗时且昂贵的过程。计算方法可用于研究目前正在进行临床试验的候选药物的特性,以确定其靶向α-syn的理论效率。单克隆抗体(mAb)是一种具有高度特异性的生物药物,Prasinezumab(PRX002)是一种目前处于II期临床的mAb,它靶向α-syn的C端(AA 118-126)。我们利用 BioLuminate 和 PyMol 对 PRX002 的片段抗原结合(Fab)区和 α-syn 的 34 种不同构象进行了结构预测和制备。使用 PIPER 进行了蛋白质-蛋白质对接模拟,并根据最佳拟合选择了 3 个对接姿势。对一式三份的对接蛋白质结构进行了 1000 ns 的分子动力学模拟,并使用 MDAnalysis 分析了氢键、静电和疏水相互作用,以确定哪些残基发生了相互作用以及相互作用的频率。结果表明,PRX002 的 HCDR2 区域与 α-syn 之间经常形成氢键。通过计算自由能确定了结合亲和力。预测的结合亲和力显示 PRX002 和 α-syn 之间具有很强的抗体抗原吸引力。计算了 RMSD,以确定这些区域在整个模拟过程中的构象变化。利用计算筛选方法确定了 mAb 的可开发性。我们的研究结果证明了这种治疗药物的有效性和可开发性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating the Mechanisms of Antibody Binding to Alpha-Synuclein for the Treatment of Parkinson’s Disease
Parkinson’s disease (PD) is an idiopathic neurodegenerative disorder with the second-highest prevalence rate behind Alzheimer’s disease. The pathophysiological hallmarks of PD are both degeneration of dopaminergic neurons in the substantia nigra pars compacta and the inclusion of misfolded α-synuclein (α-syn) aggregates known as Lewy bodies. Despite decades of research for potential PD treatments, none have been developed, and developing new therapeutic agents is a time-consuming and expensive process. Computational methods can be used to investigate the properties of drug candidates currently undergoing clinical trials to determine their theoretical efficiency at targeting α-syn. Monoclonal antibodies (mAbs) are biological drugs with high specificity, and Prasinezumab (PRX002) is an mAb currently in Phase II, which targets the C-terminus (AA 118–126) of α-syn. We utilized BioLuminate and PyMol for the structure prediction and preparation of the fragment antigen-binding (Fab) region of PRX002 and 34 different conformations of α-syn. Protein–protein docking simulations were performed using PIPER, and 3 of the docking poses were selected based on the best fit. Molecular dynamics simulations were conducted on the docked protein structures in triplicate for 1000 ns, and hydrogen bonds and electrostatic and hydrophobic interactions were analyzed using MDAnalysis to determine which residues were interacting and how often. Hydrogen bonds were shown to form frequently between the HCDR2 region of PRX002 and α-syn. Free energy was calculated to determine the binding affinity. The predicted binding affinity shows a strong antibody–antigen attraction between PRX002 and α-syn. RMSD was calculated to determine the conformational change of these regions throughout the simulation. The mAb’s developability was determined using computational screening methods. Our results demonstrate the efficiency and developability of this therapeutic agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


