揭示聚对苯二甲酸乙二醇酯 (PET) 水解酶设计中稳定性和灵活性之间的相互作用
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
聚对苯二甲酸乙二醇酯(PET)是一种广泛用于包装和纺织品的聚酯塑料,其积累已导致全球环境危机。生物降解为 PET 回收利用提供了一种前景广阔的策略,PET 水解酶(PETase)可在分子水平上完成这一任务。遗憾的是,PET 酶只能在环境温度下工作,效率较低,限制了其工业应用。目前的工程研究重点是提高 PET 酶的耐热性,但稳定性的提高会降低底物结合所需的结构动态,从而可能减缓酶的活性。为了阐明在优化 PETase 催化活性时稳定性和灵活性之间的平衡,我们利用分子动力学模拟和挫折分析对野生型 PETase(WT-PETase)和嗜热变体(Thermo-PETase)进行了理论研究。尽管最初设计的目的是为了稳定酶的原生结构,但我们的研究结果表明,与 WT-PETase 相比,Thermo-PETase 在 PET 结合位点和催化位点的结构灵活性有了前所未有的提高,有利于底物招募和产物释放。在 PET 结合时,我们观察到 Thermo-PETase 的结构动态在很大程度上被淬灭,有利于催化残基与 PET 底物的羰基之间的接近。与 WT-PETase 相比,这可能有助于提高 Thermo-PETase 发生催化反应的概率。我们认为,与 WT-PETase 相比,Thermo-PETase 可以分别利用其在高温和低温下的稳定性和灵活性,在广泛的温度范围内表现出更高的 PET 降解性能。我们的研究结果为了解 PETase 如何通过平衡稳定性和灵活性来优化其酶解性能提供了宝贵的见解,这可能有助于未来 PETase 的设计策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
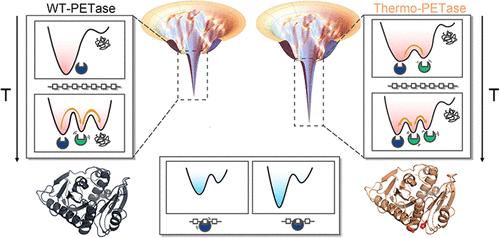
Unraveling the Interplay between Stability and Flexibility in the Design of Polyethylene Terephthalate (PET) Hydrolases
The accumulation of polyethylene terephthalate (PET), a widely used polyester plastic in packaging and textiles, has led to a global environmental crisis. Biodegradation presents a promising strategy for PET recycling, with PET hydrolases (PETase) undertaking the task at the molecular level. Unfortunately, PETase operates only at ambient temperatures with low efficiency, limiting its industrial application. Current engineering efforts focus on enhancing the thermostability of PETase, but increased stability can reduce the structural dynamics needed for substrate binding, potentially slowing enzymatic activity. To elucidate the balance between stability and flexibility in optimizing PETase catalytic activity, we performed theoretical investigations on both wild-type PETase (WT-PETase) and a thermophilic variant (Thermo-PETase) using molecular dynamics simulations and frustration analysis. Despite being initially designed to stabilize the native structure of the enzyme, our findings reveal that Thermo-PETase exhibits an unprecedented increase in structural flexibility at the PET-binding and catalytic sites, beneficial for substrate recruitment and product release, compared to WT-PETase. Upon PET binding, we observed that the structural dynamics of Thermo-PETase is largely quenched, favoring the proximity between the catalytic residues and the carbonyl of the PET substrate. This may potentially contribute to a higher probability of a catalytic reaction occurring in Thermo-PETase compared to WT-PETase. We suggest that Thermo-PETase can exhibit higher PET-degradation performance than WT-PETase across a broad temperature range by leveraging stability and flexibility at high and low temperatures, respectively. Our findings provide valuable insights into how PETase optimizes its enzymatic performance by balancing stability and flexibility, which may contribute to future PETase design strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


