Y2Ti2O7 和 Eu2Ti2O7 在 7-1800 K 温度范围内的热力学性质
IF 0.7
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
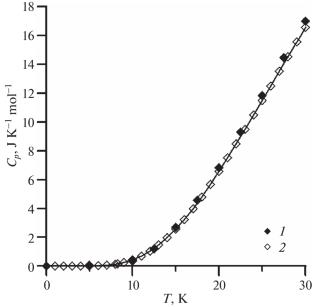
摘要 研究了具有热绿球结构的 Y2Ti2O7 和 Eu2Ti2O7 在 7-1800 K 温度范围内热容量的温度依赖性。证实了钛酸铕的热容量在 10-60 K 范围内存在较小的浅层异常。对热力学性质(熵、焓变和还原吉布斯能)进行了计算。根据氧化物形成钛酸盐的吉布斯能计算结果得出结论,这两种钛酸盐在高温区热力学上是稳定的。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Thermodynamic Properties of Y2Ti2O7 and Eu2Ti2O7 in the Temperature Range 7–1800 K
The temperature dependence of the heat capacity of Y2Ti2O7 and Eu2Ti2O7 with a pyrochlore structure in the temperature range of 7–1800 K has been studied. The existence of a small shallow anomaly of the heat capacity of europium titanate in the range of 10–60 K was confirmed. The thermodynamic properties (entropy, enthalpy change, and reduced Gibbs energy) were calculated. Based on the results of calculation of the Gibbs energy of formation of the titanates from oxides it was concluded that both titanates are thermodynamically stable in the high temperature region.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


