通过组成型表达 tHMGA2 使体内 HDAd 转导的造血干细胞自动扩增
IF 4.7
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-13
DOI:10.1016/j.omtm.2024.101319
引用次数: 0
摘要
我们开发了一种无需细胞移植的造血干细胞(HSC)基因治疗方法。为了获得治疗相关数量的校正细胞,我们构建了表达3′UTR截短HMGA2基因和GFP报告基因的造血干细胞转HDAd5/35++载体。SB100x转座酶载体介导了tHMGA2/GFP转基因盒的随机整合。通过皮下注射 G-CSF 和 AMD3100/Plerixafor 动员小鼠的造血干细胞,然后静脉注射整合的 tHMGA2/GFP 载体。这导致了 GFP PBMC 缓慢但渐进的竞争性扩增,在第 44 周达到约 50%,并在二次受体中进一步扩增。扩增发生在造血干细胞水平以及骨髓和脾脏中的髓系、淋巴系和红系祖细胞水平。重要的是,根据全基因组整合位点分析,扩增是多克隆性的,没有任何克隆优势的迹象。全外显子组测序结果显示,tHGMGA2/GFP小鼠与未经处理的对照组小鼠之间的基因组不稳定性指数没有明显差异。tHMGA2的自动扩增功能在人源化小鼠中得到了验证。这是首次证明,只需注射动员药物和 HDAd 载体,就能引发转导的造血干细胞自动扩增,从而产生转基因标记率,理论上可治疗血红蛋白病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
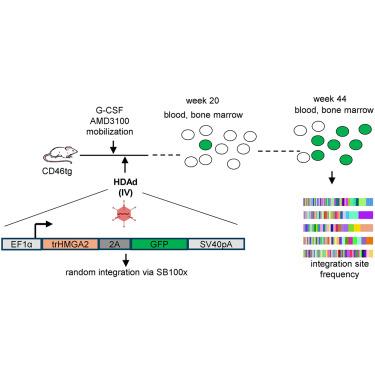
Auto-expansion of in vivo HDAd-transduced hematopoietic stem cells by constitutive expression of tHMGA2
We developed an hematopoietic stem cell (HSC) gene therapy approach that does not require cell transplantation. To achieve therapeutically relevant numbers of corrected cells, we constructed HSC-tropic HDAd5/35++ vectors expressing a 3′ UTR truncated HMGA2 gene and a GFP reporter gene. A SB100x transposase vector mediated random integration of the tHMGA2/GFP transgene cassette. HSCs in mice were mobilized by subcutaneous injections of G-CSF and AMD3100/Plerixafor and intravenously injected with the integrating tHMGA2/GFP vector. This resulted in a slow but progressive, competitive expansion of GFP PBMCs, reaching about 50% by week 44 with further expansion in secondary recipients. Expansion occurred at the level of HSCs as well as at the levels of myeloid, lymphoid, and erythroid progenitors within the bone marrow and spleen. Importantly, based on genome-wide integration site analyses, expansion was polyclonal, without any signs of clonal dominance. Whole-exome sequencing did not show significant differences in the genomic instability indices between tHGMGA2/GFP mice and untreated control mice. Auto-expansion by tHMGA2 was validated in humanized mice. This is the first demonstration that simple injections of mobilization drugs and HDAd vectors can trigger auto-expansion of transduced HSCs resulting in transgene-marking rates that, theoretically, are curative for hemoglobinopathies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


