用毛细管电泳法评估腺相关病毒的纯度
IF 4.7
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-14
DOI:10.1016/j.omtm.2024.101321
引用次数: 0
摘要
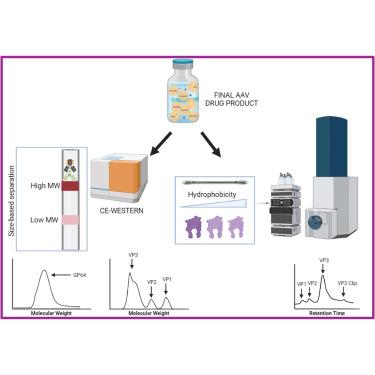
对以重组腺相关病毒(rAAV)为基础的药物产品进行严格的分析评估,是将其成功开发为临床候选药物的关键。尤其重要的是,既要确保高纯度,又要确保最终药物产品中杂质含量低。评估 rAAV 药物产品纯度的一种方法是确定组成 rAAV 包膜的三种病毒蛋白 (VP) 的相对化学计量以及最终药物产品中的杂质含量。在此,我们介绍了两种毛细管电泳-西法(CE-Western)检测方法,分别用于量化(1)使用抗 AAV B1 抗体检测 VP 的相对配比,以及(2)使用抗 GP64 抗体检测杆状病毒蛋白杂质 GP64 的残留水平。在每种检测方法中,都分析了来自不同 AAV 血清型的各种纯化样本,以确定它们的 VP 比率或 GP64 水平。rAAV 样品中 VP3/VP1 的比率与生物活性相关,并证明了 GP64 在生产过程中的清除率。液相色谱-质谱分析进一步证实了这两种检测方法得出的结果。总之,我们报告称 CE-western 是一种高通量平台,可利用低样品量快速、灵敏、稳健地评估 rAAV 药物产品的特性、成分和纯度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Assessment of adeno-associated virus purity by capillary electrophoresis-based western
A rigorous analytical assessment of recombinant adeno-associated virus (rAAV)-based drug products is critical for their successful development as clinical candidates. It is especially important to ascertain high purity while simultaneously ensuring low levels of impurities in the final drug product. One approach to evaluate the purity of rAAV drug products is to determine the relative stoichiometry of the three viral proteins (VPs) that comprise an rAAV capsid, and the levels of impurities in the final drug product. Here we present two capillary electrophoresis-western (CE-western) assays for quantifying (1) the relative stoichiometry of VP using the anti-AAV B1 antibody, and (2) residual levels of a baculovirus protein impurity, GP64, using the anti-GP64 antibody. In each assay, various purified samples from diverse AAV serotypes were analyzed to determine their VP ratio or GP64 levels. The ratio of VP3/VP1 in rAAV samples was correlated with biological activity, and the clearance of GP64 from the manufacturing process was demonstrated. The results obtained from both assays were further supported by liquid chromatography-mass spectrometry analyses. Overall, we report that CE-western is a high-throughput platform that utilizes low sample volumes for a rapid, sensitive, and robust assessment of the identity, composition, and purity of rAAV drug products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


