使用放射性标记的 AAV 载体量化小鼠腺相关病毒基因疗法的生物分布和转导的新方法
IF 4.7
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-08-19
DOI:10.1016/j.omtm.2024.101326
引用次数: 0
摘要
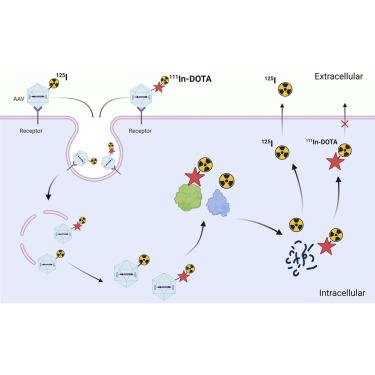
了解重组腺相关病毒(AAV)的生物分布特征是临床前开发计划的一个重要因素。在这里,我们开发了一种放射性标记策略,利用 I(非残留)和 In(残留)放射性核素结合的 AAV 共同递送,在组织水平上提供详细的分布定量,区分细胞内化的 AAV(降解的,In-I)和留在细胞外基质中的 AAV(完整的,I)。这种标记方法已成功应用于作为工具分子的 AAV9 和 AAV-PHP.eB,而不会改变 AAV 的物理性质和生物活性。用其中一种放射性探针标记后,将这些分子全身注射到 C57BL/6 小鼠体内。生物分布结果表明,AAV具有快速分布特征,在早期主要位于肝脏和脾脏等高灌注器官的细胞外基质中,这导致了囊壳定量和载体基因组定量之间的差异。结果表明,I-AAV/In-AAV联合递送方法提供了一种稳健高效的分析策略,可用于研究AAV载体(包括载体基因组和蛋白囊体)在组织中的详细分布情况。这种新方法有望应用于囊壳的优化、筛选和候选先导药物的开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A novel approach to quantitate biodistribution and transduction of adeno-associated virus gene therapy using radiolabeled AAV vectors in mice
An understanding of recombinant adeno-associated virus (AAV) biodistribution profiles is an important element of a preclinical development program. Here, we have developed a radiolabeling strategy utilizing the co-delivery of I (non-residualizing) and In (residualizing) radionuclide-conjugated AAVs to provide a detailed distribution quantification at tissue level delineating between the cellular internalized AAV (degraded, In-I) and AAV remaining in the extracellular matrix (intact, I). This labeling method has been successfully applied to AAV9 and AAV-PHP.eB as tool molecules without altering the physical properties and biological activities of the AAVs. Upon labeling with either of the radioactive probes, these molecules were systemically injected into C57BL/6 mice. The biodistribution results indicate that AAVs, with a fast distribution profile, were mainly located in the extracellular matrix of highly perfused organs such as liver and spleen at early time points, leading to a difference between capsid quantification and vector genome quantification. The results suggest that the I-AAV/In-AAV co-delivery approach offers a robust and efficient analytical strategy to investigate the detailed tissue distribution of AAV vectors, including both vector genome and protein capsids. This novel method has the potential to be applied to capsid optimization, selection, and lead candidate development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


