消除定性无机分析中的 H2S 和任何硫化物试剂:长期问题的终极解决方案
IF 2.9
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
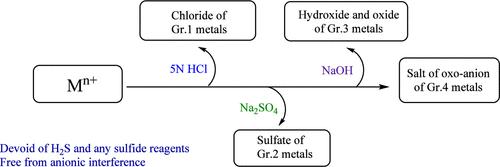
检测溶液中的金属离子无需使用 H2S 或任何其他硫化物材料。新方法不受阴离子的干扰。可以直接从样品混合物的水提取物中鉴定 Na+、K+ 和 NH4+。此外,还开发了一种无需分离基团即可直接鉴定 Mg2+ 的技术。用 5 N HCl、Na2SO4 和 5 N NaOH 作为基团试剂依次处理金属离子,将其分为四组。与现有方法相比,该方法不仅速度更快,而且足够可靠。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Eliminating H2S and Any Sulfide Reagents from Qualitative Inorganic Analysis: An Ultimate Solution to a Long-Standing Problem
Detection of metal ions in solution has been performed without employing H2S or any other sulfide materials. The new method is free from the interference from anions. Identification of Na+, K+, and NH4+ has been made possible directly from an aqueous extract of the sample mixture. A technique has been developed to identify Mg2+ directly without group separation. Metal ions have been classified into four groups by sequential treatment with 5 N HCl, Na2SO4, and 5 N NaOH as group reagents. In comparison to the existing method, it is much faster and, at the same time, reliable enough.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


