通过改变镍基氧化还原偶的电位和抑制粒子粉碎,掺杂铜提高了 LiNi0.6Mn0.2Co0.2O2 (NMC622) 的容量保持率
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
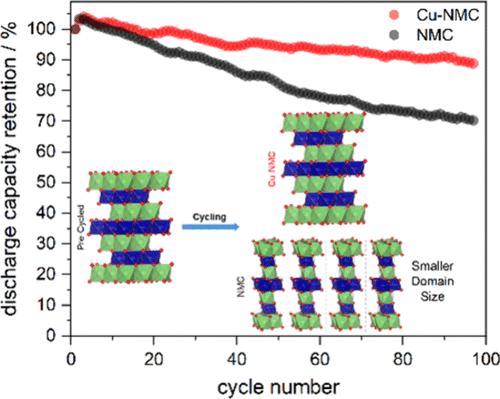
为了研究 Cu2+ 作为掺杂剂对 LiNi0.6Mn0.2Co0.2O2 (NMC622) 的结构和电化学特性的影响,在共沉淀合成过程中加入了 Cu2+(aq)。当 Cu2+ 的浓度为 5 摩尔%时,会产生单相的 Cu-NMC 产品,表现为沿 [003] 和 [104] 方向的 d 间距增大,R-3m 六方(岩盐超结构)晶格的晶体体积略有增大。XRD 数据和高分辨率 TEM 图像支持主要在过渡金属 3b Wyckoff 位点上掺入 Cu2+。Cu-NMC 的电静力循环显示出 102 mAh/g 的可逆重力容量,而未掺杂的 NMC 为 136 mAh/g。尽管容量较低,但在 100 次循环后,Cu-NMC 的放电容量保持率为 89%,而 NMC 仅为 70%。XPS 分析表明,容量降低的原因是表面 Ni3+ 离子浓度增加,而在充电顶部和底部收集的 XRD 数据显示,与 NMC(减少 74.7%)相比,Cu-NMC 的结晶畴尺寸(减少 40.5%)减少较少,这意味着二次颗粒的粉碎。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cu Doping Increases Capacity Retention in LiNi0.6Mn0.2Co0.2O2 (NMC622) by Altering the Potential of the Ni-Based Redox Couple and Inhibiting Particle Pulverization
To discern the influence of Cu2+ as a dopant on both the structural and electrochemical characteristics of LiNi0.6Mn0.2Co0.2O2 (NMC622), Cu2+(aq) was added to the coprecipitation synthesis from the constituent ions. At 5 mol % Cu2+, a single-phase Cu-NMC product results, evidenced by an increase in d-spacing along the [003] and [104] directions and a slight increase in the crystal volume of the R–3m hexagonal (rock-salt superstructure) lattice. XRD data and high-resolution TEM imaging support Cu2+ doping primarily on the transition metal 3b Wyckoff sites. Galvanostatic cycling of Cu-NMC shows a reversible gravimetric capacity of 102 mAh/g compared to 136 mAh/g for undoped NMC. Despite the lower capacity, the discharge capacity retention of Cu-NMC is 89% after 100 cycles compared to only 70% for NMC. XPS analysis reveals that this lower capacity is due to an increase in the concentration of Ni3+ ions at the surface, while XRD data collected at the top and bottom of charge show a smaller decrease in crystalline domain size for Cu-NMC (40.5% decrease) compared to NMC (74.7% decrease), translating to pulverization of the secondary particles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


