一种基于鲨鱼 VNAR 抗体、以 TROP-2 为靶点的新型免疫毒素用于癌症治疗
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
TROP-2是一种肿瘤相关抗原,与各种上皮性肿瘤的进展有关。由于其良好的表达谱,TROP-2 已成为基于抗体药物共轭物(ADCs)的抗肿瘤疗法的一个有希望的靶点。虽然 ADCs 在癌症治疗中已显示出疗效,但其在实体瘤中的应用却因分子量高、肿瘤穿透性差和释放细胞毒性分子而受到阻碍。因此,我们开发了一种基于鲨鱼来源的免疫球蛋白新抗原受体可变域(VNAR)抗体的重组免疫毒素。VNAR 的大小仅为 IgG 抗体的十分之一,具有显著的组织穿透能力和高稳定性。本研究创建了鲨鱼 VNAR 噬菌体展示文库,从而鉴定出了针对 TROP-2 的鲨鱼 VNAR-5G8。VNAR-5G8 对表达高水平 TROP-2 的细胞具有高亲和力和细胞内化能力。表位分析表明,VNAR-5G8 能识别 TROP-2 上由 CRD 和 TY-1 组成的隐藏表位。随后,VNAR-5G8与截短形式的(PE38)融合,产生了重组免疫毒素(5G8-PE38),它表现出了显著的抗肿瘤活性和......。总之,这项研究强调了 5G8-PE38 作为一种有价值的候选癌症疗法的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
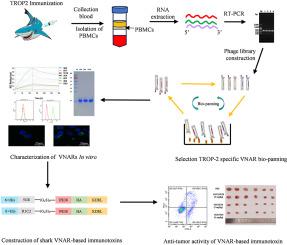
A novel shark VNAR antibody-based immunotoxin targeting TROP-2 for cancer therapy
TROP-2, a tumor-associated antigen, has been implicated in the progression of various epithelial tumors. Due to its favorable expression profile, TROP-2 has emerged as a promising target for antibody–drug conjugates (ADCs) based anti-tumor therapies. Although ADCs have shown efficacy in cancer treatment, their application in solid tumors is hindered by their high molecular weight, poor tumor penetration, and release of cytotoxic molecules. Therefore, a recombinant immunotoxin was developed based on a shark-derived variable domain of immunoglobulin new antigen receptor (VNAR) antibody. VNARs are only one-tenth the size of IgG antibodies and possess remarkable tissue penetration capabilities and high stability. In this study, a shark VNAR phage display library was created, leading to the identification of shark VNAR-5G8 that targets TROP-2. VNAR-5G8 exhibited a high affinity and cellular internalization ability towards cells expressing high levels of TROP-2. Epitope analysis revealed that VNAR-5G8 recognizes a hidden epitope consisting of CRD and TY-1 on TROP-2. Subsequently, VNAR-5G8 was fused with a truncated form of Pseudomonas exotoxin (PE38) to create the recombinant immunotoxin (5G8-PE38), which exhibited significant anti-tumor activity in vitro and in vivo. Overall, this study highlights the promise of 5G8-PE38 as a valuable candidate for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


