通过转录激活脂肪酸结合蛋白 4,激活孕烷 X 受体使酒精性脂肪性肝炎变得敏感
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
酒精性脂肪性肝炎(ASH)是一种肝脏疾病,其特征是由于过量饮酒导致的肝组织脂肪变性、发炎和坏死。孕烷 X 受体(PXR)是一种异种生物核受体,因其对药物代谢和处置的转录调控功能而最为人熟知。临床报告显示,抗生素利福平(一种强效的人类 PXR 激活剂)是酗酒者的禁忌药物,但其机制尚不清楚。在这项研究中,我们发现在 ASH 患者和 ASH 小鼠模型中,脂肪酸结合蛋白 4(FABP4)的肝脏表达独特地升高。药物抑制 FABP4 可减轻小鼠的 ASH。此外,用小鼠 PXR 激动剂孕烯诺龙-16-甲腈(PCN)处理小鼠会诱导肝脏和循环中的 FABP4 水平,并以 PXR 依赖性方式加剧 ASH。我们的机制研究确定了 FABP4 是 PXR 的转录靶标。穿心莲内酯是一种天然化合物,也是 PXR 和 FABP4 的双重抑制剂。总之,我们的研究结果表明,PXR-FABP4基因调控轴在ASH的进展中起着重要作用,这可能是酒精性肝病患者禁用利福平的原因。药物抑制PXR和/或FABP4可能有望用于ASH的临床治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
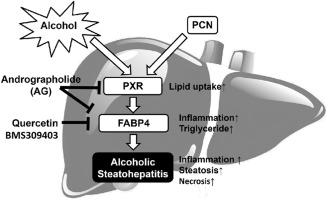
Activation of pregnane X receptor sensitizes alcoholic steatohepatitis by transactivating fatty acid binding protein 4
Alcoholic steatohepatitis (ASH) is a liver disease characterized by steatosis, inflammation, and necrosis of the liver tissue as a result of excessive alcohol consumption. Pregnane X receptor (PXR) is a xenobiotic nuclear receptor best known for its function in the transcriptional regulation of drug metabolism and disposition. Clinical reports suggested that the antibiotic rifampicin, a potent human PXR activator, is a contraindication in alcoholics, but the mechanism was unclear. In this study, we showed that the hepatic expression of fatty acid binding protein 4 (FABP4) was uniquely elevated in ASH patients and a mouse model of ASH. Pharmacological inhibiting FABP4 attenuated ASH in mice. Furthermore, treatment of mice with the mouse PXR agonist pregnenolon-16α-carbonitrile (PCN) induced the hepatic and circulating levels of FABP4 and exacerbated ASH in a PXR-dependent manner. Our mechanism study established FABP4 as a transcriptional target of PXR. Treatment with andrographolide, a natural compound and dual inhibitor of PXR and FABP4, alleviated mice from ASH. In summary, our results showed that the PXR–FABP4 gene regulatory axis plays an important role in the progression of ASH, which may have accounted for the contraindication of rifampicin in patients of alcoholic liver disease. Pharmacological inhibition of PXR and/or FABP4 may have its promise in the clinical management of ASH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


