去甲斑蝥素诱导 PD-L1 泛素-蛋白酶体降解,并通过靶向 USP22 促进抗肿瘤免疫力
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
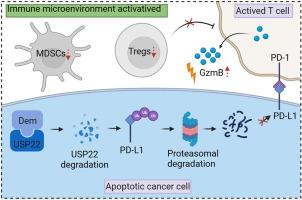
程序性细胞死亡配体-1(PD-L1)是一种抑制T细胞的免疫检查点分子,它与程序性细胞死亡-1(PD-1)相互作用,促进肿瘤细胞的免疫逃逸。与抗体疗法相比,小分子药物因其生物利用度高、组织穿透性好、免疫原性风险低等优势而显示出更好的前景。在这里,我们发现小分子去甲斑蝥素(Dem)能显著下调结直肠癌细胞中PD-L1的表达,增强T细胞对肿瘤细胞的杀伤作用。从机理上讲,Dem 可与去泛素化酶 USP22 结合并促进其降解,从而增加 PD-L1 通过蛋白酶体途径的泛素化和降解。此外,Dem还能提高细胞毒性T细胞的活性,减少肿瘤浸润淋巴细胞(TILs)中髓源性抑制细胞(MDSCs)和调节性T细胞(Tregs)的数量,从而激活肿瘤免疫微环境,抑制C57BL/6小鼠皮下MC38肿瘤的生长。此外,我们还发现Dem和CTLA4抗体联合使用能进一步提高抗肿瘤治疗的疗效。我们的研究揭示了Dem促进PD-L1降解的机制,并提示Dem和CTLA4抗体联合使用可提高免疫疗法的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Demethylzeylasteral induces PD-L1 ubiquitin–proteasome degradation and promotes antitumor immunity via targeting USP22
Programmed cell death ligand-1 (PD-L1) is a T cell inhibitory immune checkpoint molecule that interacts with programmed cell death-1 (PD-1) to promote immune escape of tumor cells. Compared with antibody therapies, small molecule drugs show better prospects due to their advantages such as higher bioavailability, better tissue penetration, and reduced risk of immunogenicity. Here, we found that the small molecule demethylzeylasteral (Dem) can significantly downregulate the expression of PD-L1 in colorectal cancer cells and enhance the killing effect of T cells on tumor cells. Mechanistically, Dem binds to the deubiquitinating enzyme USP22 and promotes its degradation, resulting in increased ubiquitination and degradation of PD-L1 through the proteasome pathway. In addition, Dem increased the activity of cytotoxic T cells and reduced the number of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in tumor-infiltrating lymphocytes (TILs), thereby activating the tumor immune microenvironment and inhibiting the growth of subcutaneous MC38 tumors in C57BL/6 mice. Moreover, we also found that the combination of Dem and CTLA4 antibodies can further improve the efficacy of antitumor therapy. Our study reveals the mechanism by which Dem promotes PD-L1 degradation and suggests that the combination of Dem and CTLA4 antibodies may improve the efficacy of immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


