筛选与转甲状腺素结合的 ToxCast 化学库
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
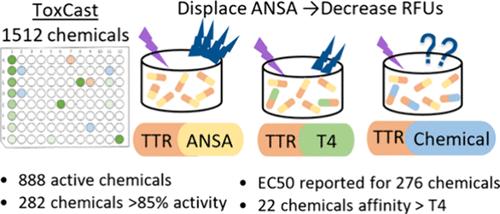
转甲状腺素(TTR)是一种血清结合蛋白,负责将甲状腺激素(TH)运输到目标组织并维持可用甲状腺激素的平衡。化学物质与 TTR 的结合以及随后 TH 的转移已被确定为筛选可能破坏甲状腺系统的化学物质的终点。为了解决缺乏化学品与 TTR 结合数据的问题,我们优化了一种体外检测方法,利用荧光探针 8-苯胺基-1-萘磺酸 (ANSA) 和人类蛋白质 TTR,采用分层方法从美国环保署的 ToxCast ph1_v2、ph2 和 e1k 库中筛选出 1500 多种化学品。对单一高浓度(目标值 100 μM)进行测试后,根据 ANSA 与 TTR 的置换关系,888 种化学品的活性达到或超过 20%。其中,282 种化学品的活性达到或超过 85%,并在目标浓度为 0.015 至 100 μM 的 12 点浓度反应中进行了进一步测试。在这 301 种化学物质中,有 276 种获得了 EC50 值。迄今为止,这是筛选出的与 TTR 结合的最大一组化学物质。利用这种检测方法对扩大体外检测的范围,以确定可能破坏甲状腺激素平衡的化学物质做出了重大贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Screening the ToxCast Chemical Libraries for Binding to Transthyretin
Transthyretin (TTR) is one of the serum binding proteins responsible for transport of thyroid hormones (TH) to target tissue and for maintaining the balance of available TH. Chemical binding to TTR and subsequent displacement of TH has been identified as an end point in screening chemicals for potential disruption of the thyroid system. To address the lack of data regarding chemicals binding to TTR, we optimized an in vitro assay utilizing the fluorescent probe 8-anilino-1-napthalenesulfonic acid (ANSA) and the human protein TTR to screen over 1500 chemicals from the U.S. EPA’s ToxCast ph1_v2, ph2, and e1k libraries utilizing a tiered approach. Testing of a single high concentration (target 100 μM) resulted in 888 chemicals with 20% or greater activity based on displacement of ANSA from TTR. Of these, 282 chemicals had activity of 85% or greater and were further tested in 12-point concentration–response with target concentrations ranging from 0.015 to 100 μM. An EC50 was obtained for 276 of these 301 chemicals. To date, this is the largest set of chemicals screened for binding to TTR. Utilization of this assay is a significant contribution toward expanding the suite of in vitro assays used to identify chemicals with the potential to disrupt thyroid hormone homeostasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.30%
发文量
215
审稿时长
3.5 months
期刊介绍:
Chemical Research in Toxicology publishes Articles, Rapid Reports, Chemical Profiles, Reviews, Perspectives, Letters to the Editor, and ToxWatch on a wide range of topics in Toxicology that inform a chemical and molecular understanding and capacity to predict biological outcomes on the basis of structures and processes. The overarching goal of activities reported in the Journal are to provide knowledge and innovative approaches needed to promote intelligent solutions for human safety and ecosystem preservation. The journal emphasizes insight concerning mechanisms of toxicity over phenomenological observations. It upholds rigorous chemical, physical and mathematical standards for characterization and application of modern techniques.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


