小分子 NS11021 通过增加内孔水合作用促进 BK 通道活化
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
钙离子通道和电压门控大钾(BK)通道与心脏病、哮喘、癫痫和癌症等多种疾病有关,但仍然是难以捉摸的药物靶点。一类带负电荷的激活剂(NCAs)已被证明能通过与疏水性内孔结合促进包括 BK 通道在内的多种钾通道的激活,但其潜在的分子作用机制仍鲜为人知。在这项工作中,我们利用原子模拟分析了一种名为 NS11021 的特定 NCA 的结合模式和潜在激活机制。结果表明,NS11021 与失活 BK 通道孔的结合是非特异性和动态的。利用伞状取样计算出的结合自由能为 -8.3 ± 0.7 kcal/mol(KD = 0.3-3.1 μM),与实验 EC50 的 0.4-2.1 μM 范围定量吻合。结合的 NS11021 保持动态,与过滤器的距离较远,不会对其构象产生显著影响。相反,由于带电的四唑苯基基团,NS11021 的结合会显著增强孔隙的水合作用,从而促进疏水门的打开。我们还进一步发现,无论以何种姿势结合,K+渗透的自由能障都会降低 3 千卡/摩尔,这就可以解释为什么实验测得的 BK 通道内在开放程度提高了 62 倍。综上所述,这些结果支持这样一种观点,即 NS11021 的分子机制源于提高构象封闭孔道的水合水平,这并不依赖于特异性结合,而且很可能解释了 NCA 激活多个 K+ 通道的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
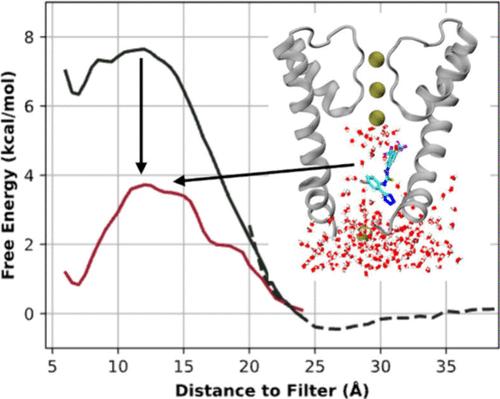
Small Molecule NS11021 Promotes BK Channel Activation by Increasing Inner Pore Hydration
The Ca2+ and voltage-gated big potassium (BK) channels are implicated in various diseases, including heart disease, asthma, epilepsy, and cancer, but remain an elusive drug target. A class of negatively charged activators (NCAs) have been demonstrated to promote the activation of several potassium channels including BK channels by binding to the hydrophobic inner pore, yet the underlying molecular mechanism of action remains poorly understood. In this work, we analyze the binding mode and potential activation mechanism of a specific NCA named NS11021 using atomistic simulations. The results show that NS11021 binding to the pore in deactivated BK channels is nonspecific and dynamic. The binding free energy of −8.3 ± 0.7 kcal/mol (KD = 0.3–3.1 μM) calculated using umbrella sampling agrees quantitatively with the experimental EC50 range of 0.4–2.1 μM. The bound NS11021 remains dynamic and is distal from the filter to significantly impact its conformation. Instead, NS11021 binding significantly enhances the pore hydration due to the charged tetrazol-phenyl group, thereby promoting the opening of the hydrophobic gate. We further show that the free energy barrier to K+ permeation is reduced by ∼3 kcal/mol regardless of the binding pose, which could explain the ∼62-fold increase in the intrinsic opening of BK channels measured experimentally. Taken together, these results support the idea that the molecular mechanism of NS11021 derives from increasing the hydration level of the conformationally closed pore, which does not depend on specific binding and likely explains the ability of NCAs to activate multiple K+ channels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


