通过芳香族氨基酸抑制核-细胞质蛋白酶体转运或沉默Sestrin3--它们的感应介质--具有抑瘤作用
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
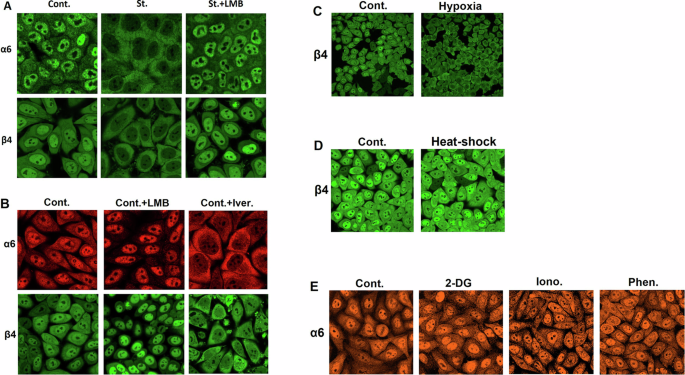
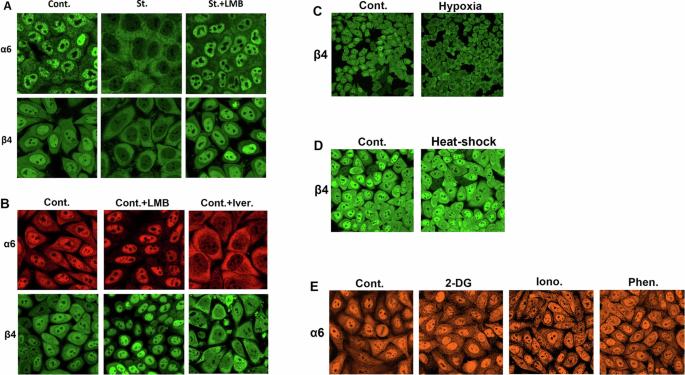
蛋白酶体是泛素系统的催化臂,通过其在细胞核和细胞质之间的动态分区等机制进行调节。在氨基酸短缺的情况下,蛋白水解复合物会转运到细胞质中,在那里刺激蛋白水解,以补充用于合成必需蛋白质的再生氨基酸。这种反应是通过 mTOR 途径和三种芳香族氨基酸 Tyr、Trp 和 Phe(YWF)的缺乏来介导的。通过补充三元组来激活 mTOR 可抑制蛋白酶体的转运,从而导致细胞死亡。我们现在证明,肿瘤固有的应激条件会导致蛋白酶体从细胞核转位到细胞质。我们还进一步证明,通过使用更高浓度的三聚氰胺来实现相对于所有其他氨基酸的过剩,YWF 对信号级联的调节也适用于非饥饿细胞。基于这两种现象,我们发现通过服用 YWF 调节应激信号可导致核蛋白酶体螯合,并抑制异种移植、自发性和转移性小鼠肿瘤模型的生长。与观察到的 YWF 对肿瘤的影响相关,我们通过转录组和蛋白质组分析发现,三联体影响了与细胞增殖、迁移和死亡相关的各种细胞过程。此外,Sestrin3--mTOR上游YWF感应的介质--对蛋白酶体转运至关重要,因此起着促癌作用,被定位为潜在的致癌基因。因此,这种新发现的劫持细胞 "饱食中心 "的方法对癌症具有潜在的治疗意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhibition of nucleo-cytoplasmic proteasome translocation by the aromatic amino acids or silencing Sestrin3—their sensing mediator—is tumor suppressive
The proteasome, the catalytic arm of the ubiquitin system, is regulated via its dynamic compartmentation between the nucleus and the cytoplasm, among other mechanisms. Under amino acid shortage, the proteolytic complex is translocated to the cytoplasm, where it stimulates proteolysis to supplement recycled amino acids for essential protein synthesis. This response is mediated via the mTOR pathway and the lack of the three aromatic amino acids Tyr, Trp, and Phe (YWF). mTOR activation by supplementation of the triad inhibits proteasome translocation, leading to cell death. We now show that tumoral inherent stress conditions result in translocation of the proteasome from the nucleus to the cytosol. We further show that the modulation of the signaling cascade governed by YWF is applicable also to non-starved cells by using higher concentration of the triad to achieve a surplus relative to all other amino acids. Based on these two phenomena, we found that the modulation of stress signals via the administration of YWF leads to nuclear proteasome sequestration and inhibition of growth of xenograft, spontaneous, and metastatic mouse tumor models. In correlation with the observed effect of YWF on tumors, we found – using transcriptomic and proteomic analyses – that the triad affects various cellular processes related to cell proliferation, migration, and death. In addition, Sestrin3—a mediator of YWF sensing upstream of mTOR—is essential for proteasome translocation, and therefore plays a pro-tumorigenic role, positioning it as a potential oncogene. This newly identified approach for hijacking the cellular “satiety center” carries therefore potential therapeutic implications for cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


