在线粒体上组织功能性糖酵解代谢子以提高代谢效率
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
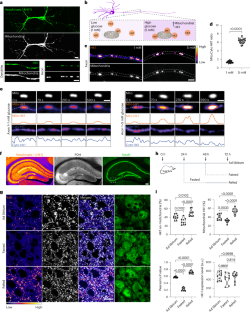
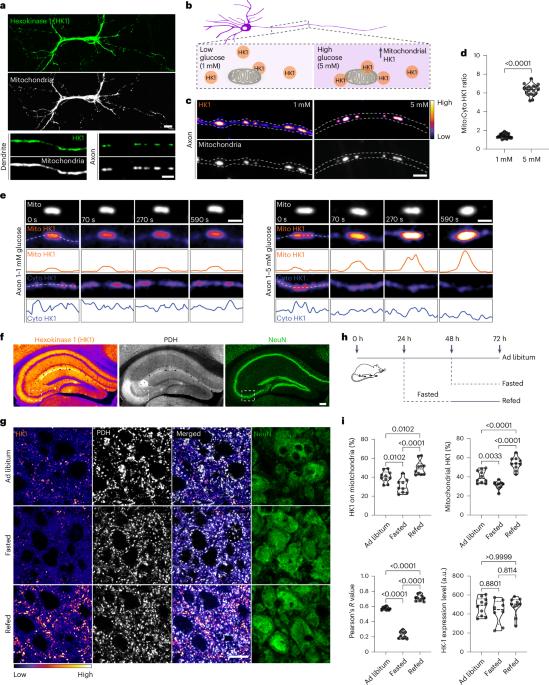
葡萄糖是细胞的主要能量来源,通过限速酶六磷酸酶(HK)启动的糖酵解进行代谢。在大脑等需要能量的组织中,HK1 是主要的同工酶,主要定位于线粒体上,对于有效的糖酵解-氧化磷酸化耦合和最佳能量生成至关重要。这项研究揭示了一种调节 HK1 活性、糖酵解和线粒体耦合动态的独特机制,该机制由代谢传感器酶 O-GlcNAc 转移酶(OGT)介导。OGT 催化可逆的 O-GlcNAcylation,这是一种受葡萄糖通量影响的翻译后修饰。OGT 活性的升高会诱导 HK1 的调节结构域发生动态的 O-GlcNAcyl 化,从而促进糖酵解代谢子在线粒体外膜上的组装。这种修饰增强了线粒体与 HK1 的结合,协调了糖酵解和线粒体 ATP 的产生。HK1 的 O-GlcNAcylation 位点突变会减少多种细胞类型的 ATP 生成,尤其会影响神经元的代谢效率。这项研究揭示了一种以前未被重视的途径,它通过 OGT 和糖酵解代谢子的形成将神经元代谢和线粒体功能联系起来,为解决代谢和神经系统疾病提供了潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency
Glucose, the primary cellular energy source, is metabolized through glycolysis initiated by the rate-limiting enzyme hexokinase (HK). In energy-demanding tissues like the brain, HK1 is the dominant isoform, primarily localized on mitochondria, and is crucial for efficient glycolysis–oxidative phosphorylation coupling and optimal energy generation. This study unveils a unique mechanism regulating HK1 activity, glycolysis and the dynamics of mitochondrial coupling, mediated by the metabolic sensor enzyme O-GlcNAc transferase (OGT). OGT catalyses reversible O-GlcNAcylation, a post-translational modification influenced by glucose flux. Elevated OGT activity induces dynamic O-GlcNAcylation of the regulatory domain of HK1, subsequently promoting the assembly of the glycolytic metabolon on the outer mitochondrial membrane. This modification enhances the mitochondrial association with HK1, orchestrating glycolytic and mitochondrial ATP production. Mutation in HK1’s O-GlcNAcylation site reduces ATP generation in multiple cell types, specifically affecting metabolic efficiency in neurons. This study reveals a previously unappreciated pathway that links neuronal metabolism and mitochondrial function through OGT and the formation of the glycolytic metabolon, providing potential strategies for tackling metabolic and neurological disorders. Wang et al. show how glucose sensing via O-GlcNAcylation drives the assembly of a glycolytic metabolon on the mitochondrial surface to couple metabolic efficiency with neuronal activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


