O-GlcNAc 转移酶调节线粒体上糖酵解代谢物的形成,以提高 ATP 的产生
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
代谢传感酶 OGT 动态地将己酮酶 1(HK1)进行 O-GlcNAcyl 化。这种修饰增强了 HK1 对葡萄糖通量的线粒体定位,促进了线粒体外膜上糖酵解代谢物的形成,从而提高了糖酵解和线粒体 ATP 的产生率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
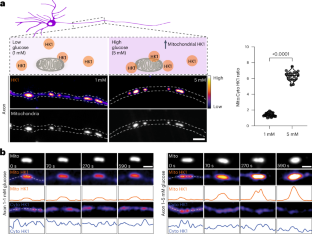
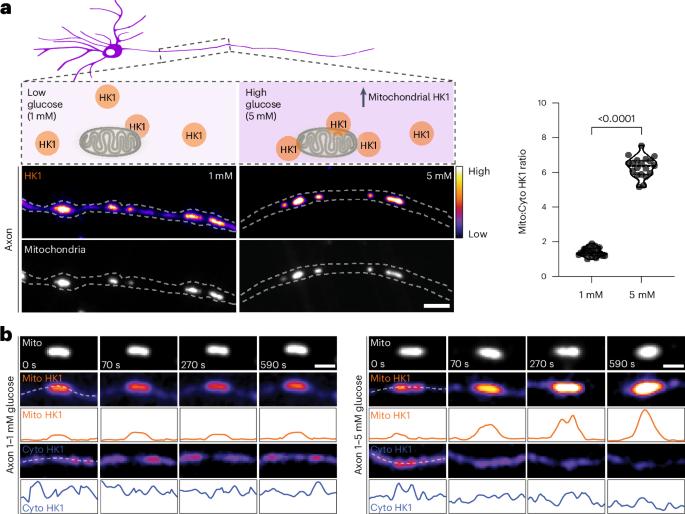
O-GlcNAc transferase regulates glycolytic metabolon formation on mitochondria to enhance ATP production
The metabolic sensor enzyme OGT dynamically O-GlcNAcylates hexokinase 1 (HK1). This modification enhances the localization of HK1 to mitochondria in response to glucose flux and facilitates the formation of a glycolytic metabolon on the mitochondrial outer membrane, leading to increased rates of both glycolytic and mitochondrial ATP production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


