针对HER2扩增或突变肿瘤的曲妥珠单抗埃坦新(Trastuzumab emtansine)信号搜索2期研究
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
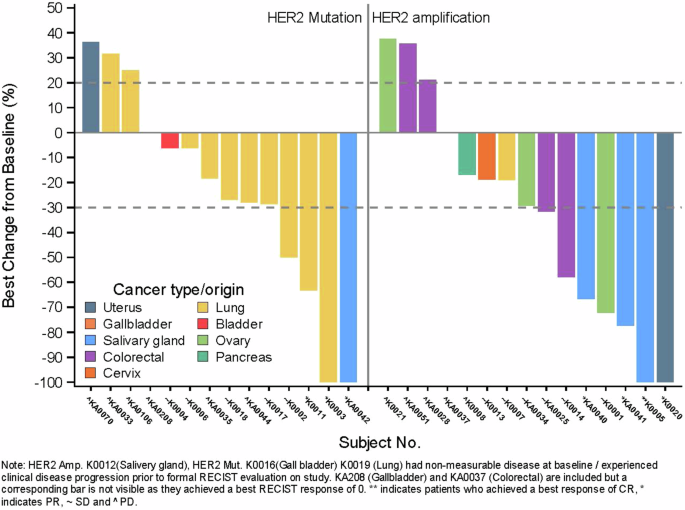
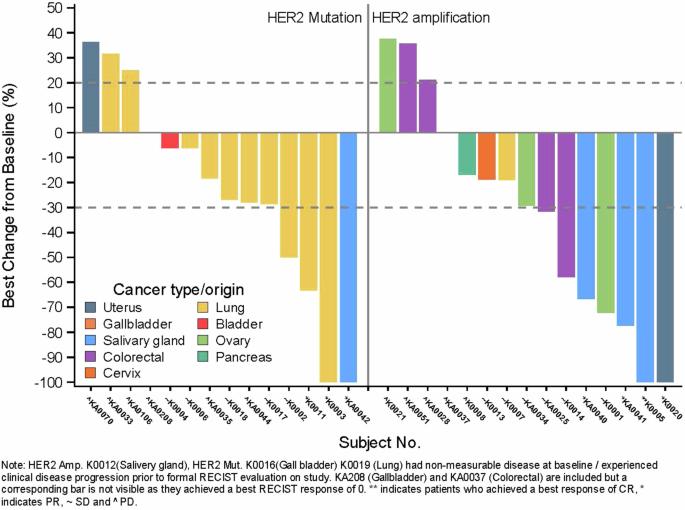
这项单臂 II 期非随机试验(ACTRN12619001265167)评估了曲妥珠单抗埃坦辛在综合基因组图谱检测到 HER2 扩增或突变的实体瘤中的应用。首要目标是客观反应(OR),次要目标包括研究进展时间(TTP)与既往治疗进展时间(TTP)之比、无进展生存期(PFS)和总生存期(OS)。队列中包括16个HER2突变肿瘤(第1组)和16个HER2扩增肿瘤(第2组)。经过17个月的中位随访,19%的第1组(1例唾液腺癌(SGC)、2例肺癌)和25%的第2组(3例SGC、1例子宫癌)发生了OR。29例TTP有效患者中有14例的TTP比值≥1.3,其中10例无OR。中位 PFS 和 OS 分别为 4.5 个月(95% CI 2.1-7.0)和 18.2 个月(95% CI 8.1-未达到)。曲妥珠单抗埃坦新(Trastuzumab emtansine)在部分HER2改变的实体瘤中显示出适度的ORs和有利的疾病轨迹变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A signal-seeking phase 2 study of Trastuzumab emtansine in tumours harbouring HER2 amplification or mutation
This single-arm phase II non-randomised trial (ACTRN12619001265167) evaluated trastuzumab emtansine in solid cancers with HER2 amplification or mutation detected by comprehensive genomic profiling. The primary objective was objective response (OR), while secondary objectives included the time to progression (TTP) on study to TTP on prior therapy ratio, progression-free survival (PFS) and overall survival (OS). The cohort included 16 tumours with HER2 mutations (group 1) and 16 with HER2 amplification (group 2). After 17 months median follow-up, ORs occurred in 19% of group 1 (1 salivary gland carcinoma (SGC), 2 lung cancers) and 25% of group 2 (3 SGCs, 1 uterine carcinoma). Fourteen of 29 TTP-evaluable patients achieved a TTP ratio ≥1.3, including 10 without an OR. Median PFS and OS were 4.5 (95% CI 2.1–7.0) and 18.2 months (95% CI 8.1-not reached) respectively. Trastuzumab emtansine showed modest ORs and a favourable change in disease trajectory in select HER2-altered solid cancers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


