PHGDH/SYK:整合抗真菌免疫和丝氨酸代谢的枢纽
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
免疫细胞在应对真菌感染时会改变其代谢途径。尽管如此,其生化基础仍有待进一步了解。本研究报告指出,真菌感染通过诱导脾酪氨酸激酶(SYK)和磷酸甘油脱氢酶(PHGDH)的相互作用,促使细胞从糖酵解转向丝氨酸合成途径(SSP)和一碳代谢。因此,PHGDH 促进 SYK 磷酸化,导致 SYK 招募到 C 型凝集素受体(CLR)。CLR/SYK 复合物启动信号级联,导致转录因子活化和促炎细胞因子的产生。SYK 通过诱导 PHGDH 的活性激活 SSP 和一碳代谢。然后,一碳代谢支持 S-腺苷蛋氨酸和组蛋白 H3 赖氨酸 36 三甲基化,从而推动促炎细胞因子和趋化因子的产生。这些发现揭示了真菌感染过程中氨基酸代谢、表观遗传修饰和 CLR 信号转导之间的相互影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
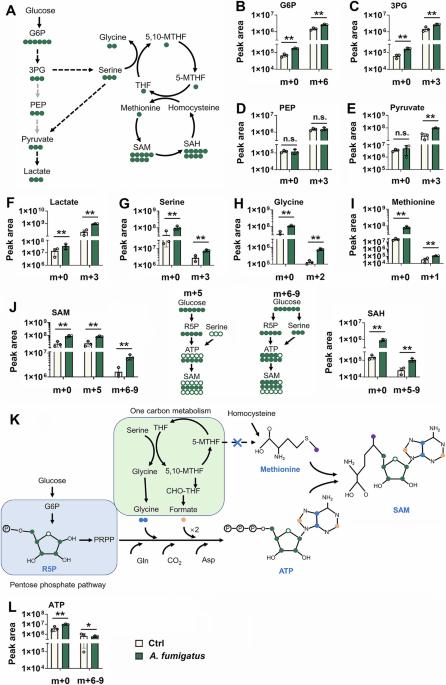
PHGDH/SYK: a hub integrating anti-fungal immunity and serine metabolism
Immune cells modify their metabolic pathways in response to fungal infections. Nevertheless, the biochemical underpinnings need to be better understood. This study reports that fungal infection drives a switch from glycolysis to the serine synthesis pathway (SSP) and one-carbon metabolism by inducing the interaction of spleen tyrosine kinase (SYK) and phosphoglycerate dehydrogenase (PHGDH). As a result, PHGDH promotes SYK phosphorylation, leading to the recruitment of SYK to C-type lectin receptors (CLRs). The CLR/SYK complex initiates signaling cascades that lead to transcription factor activation and pro-inflammatory cytokine production. SYK activates SSP and one-carbon metabolism by inducing PHGDH activity. Then, one-carbon metabolism supports S-adenosylmethionine and histone H3 lysine 36 trimethylation to drive the production of pro-inflammatory cytokines and chemokines. These findings reveal the crosstalk between amino acid metabolism, epigenetic modification, and CLR signaling during fungal infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


