间充质干细胞的细胞外囊泡能改变肠道微生物群,改善轻度肝损伤大鼠的神经炎症和运动障碍。
IF 5.6
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
有报道称,脂肪肝患者会出现肠道微生物群紊乱和运动功能障碍。轻度肝损伤(MLD)大鼠表现出由神经炎症和小脑GABA能神经传导改变介导的运动功能障碍。间充质干细胞(MSC)的细胞外囊泡(EV)已成为一种很有前景的治疗手段,其分子基础部分依赖于TGFβ的作用。本研究旨在评估间充质干细胞-EV是否能改善轻度肝损伤大鼠的运动功能障碍,并分析其潜在机制,包括TGFβ、小脑神经炎症和肠道微生物群的作用。给大鼠注射四氯化碳诱导MLD,并注射正常间充质干细胞(C-EVs)或TGFβ-siRNA处理过的间充质干细胞(T-EV)的EVs。研究人员分析了小脑的运动协调性、运动步态、神经炎症和 TNF-α 激活调节 GABA 能神经传导的途径、粪便中的微生物群组成以及血浆中的微生物衍生代谢物。C-EVs减少了神经胶质和TNFα-P2X4-BDNF-TrkB通路的激活,恢复了小脑的GABA能神经传递,改善了运动协调性和所有步态参数的改变。T-EVs也能改善运动协调性和某些步态参数,但其机制与C-EVs不同。MLD 大鼠粪便中某些乳酸菌的含量增加,这与犬尿氨酸的减少有关,但与运动变化无关。C-EVs 可使这些改变恢复正常,而 T-EVs 只能恢复犬尿氨酸的水平。我们的研究结果支持间充质干细胞-EVs在改善MLD运动功能障碍方面的价值,并揭示了微生物群改变可能导致神经炎症和运动障碍的机制。其中一些潜在机制是依赖于 TGFβ的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
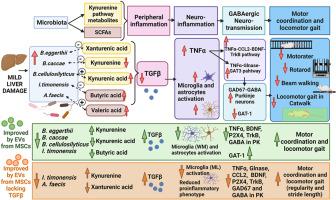
Extracellular vesicles from mesenchymal stem cells alter gut microbiota and improve neuroinflammation and motor impairment in rats with mild liver damage
Gut microbiota perturbation and motor dysfunction have been reported in steatosis patients. Rats with mild liver damage (MLD) show motor dysfunction mediated by neuroinflammation and altered GABAergic neurotransmission in the cerebellum. The extracellular vesicles (EV) from mesenchymal stem cells (MSC) have emerged as a promising therapeutic proxy whose molecular basis relies partly upon TGFβ action. This study aimed to assess if MSC-EVs improve motor dysfunction in rats with mild liver damage and analyze underlying mechanisms, including the role of TGFβ, cerebellar neuroinflammation and gut microbiota. MLD in rats was induced by carbon tetrachloride administration and EVs from normal (C-EVs) or TGFβ-siRNA treated MSCs (T-EV) were injected. Motor coordination, locomotor gait, neuroinflammation and TNF-α-activated pathways modulating GABAergic neurotransmission in the cerebellum, microbiota composition in feces and microbial-derived metabolites in plasma were analyzed. C-EVs reduced glial and TNFα-P2X4-BDNF-TrkB pathway activation restoring GABAergic neurotransmission in the cerebellum and improving motor coordination and all the altered gait parameters. T-EVs also improved motor coordination and some gait parameters, but the mechanisms involved differed from those of C-EVs. MLD rats showed increased content of some Bacteroides species in feces, correlating with decreased kynurenine aside from motor alterations. These alterations were all normalized by C-EVs, whereas T-EVs only restored kynurenine levels. Our results support the value of MSC-EVs on improving motor dysfunction in MLD and unveil a possible mechanism by which altered microbiota may contribute to neuroinflammation and motor impairment. Some of the underlying mechanisms are TGFβ-dependent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


