MyoD1 对 PINK1 的转录激活介导线粒体平衡,从而诱发小儿肾炎的肾钙化。
摘要
本研究旨在利用高通量测序数据揭示 MyoD1 对 PINK1 的调控,从而揭示肾钙沉积诱发小儿肾结石形成的分子机制。我们对健康儿童和肾结石患儿的外周血样本进行了转录组测序,以获得差异表达基因(DEGs)。我们从Genecards网站上获取了与线粒体氧化应激有关的基因,并与DEGs交叉,从而获得候选靶基因。此外,我们还利用STRING数据库进行了蛋白-蛋白相互作用(PPI)分析,以确定参与小儿肾结石病(KSD)的核心基因,并利用hTFtarget数据库预测了这些基因的转录因子。我们利用双荧光素酶报告实验评估了MyoD1对PINK1启动子活性的影响,并通过染色质免疫沉淀(ChIP)实验研究了MyoD1在PINK1启动子上的富集情况。为了验证我们的假设,我们选择了 HK-2 细胞,并建立了由一水草酸钙(COM)诱导的体外肾结石模型。我们通过测量线粒体膜电位(Δψm)、超氧化物歧化酶(SOD)活性、活性氧(ROS)和丙二醛(MDA)含量,评估了各组中各种基因的表达水平、细胞活力、附着晶体的体积以及线粒体氧化应激。利用 mtDNA 荧光染色和 PINK1 相关蛋白的 Western 印迹分析评估线粒体自噬。使用 Western 印迹分析评估了细胞凋亡相关蛋白,并使用流式细胞术测量了细胞凋亡。此外,我们还建立了一个 KSD 大鼠模型,并使用 H&E 和 von Kossa 染色法、透射电子显微镜(TEM)以及肌酐的表达评估了各种基因的表达水平以及大鼠肾组织的病理变化、通过测量Δψm、SOD 活性、ROS 和 MDA 含量,评估体内线粒体氧化应激。线粒体自噬通过对 PINK1 相关蛋白的 Western 印迹分析进行评估。通过 Western 印迹分析检测了细胞凋亡相关蛋白,并通过细胞流式细胞仪和 TUNEL 染色检测了细胞凋亡。生物信息学分析表明,PINK1基因在小儿肾结石患者中上调且具有重要意义。我们的体外和体内实验表明,通过抑制线粒体氧化应激,沉默 PINK1 可抑制肾结石的形成。我们发现MyoD1是PINK1的上游转录因子,它通过激活PINK1导致小儿肾结石的发生。我们的体内和体外实验共同证实,在肾结石大鼠模型中,沉默MyoD1可通过下调PINK1的表达,抑制线粒体氧化应激、线粒体自噬和细胞凋亡,从而抑制肾结石的形成。在这项研究中,我们发现MyoD1可通过转录激活PINK1诱导线粒体氧化应激,从而促进小儿肾结石的形成和发展。
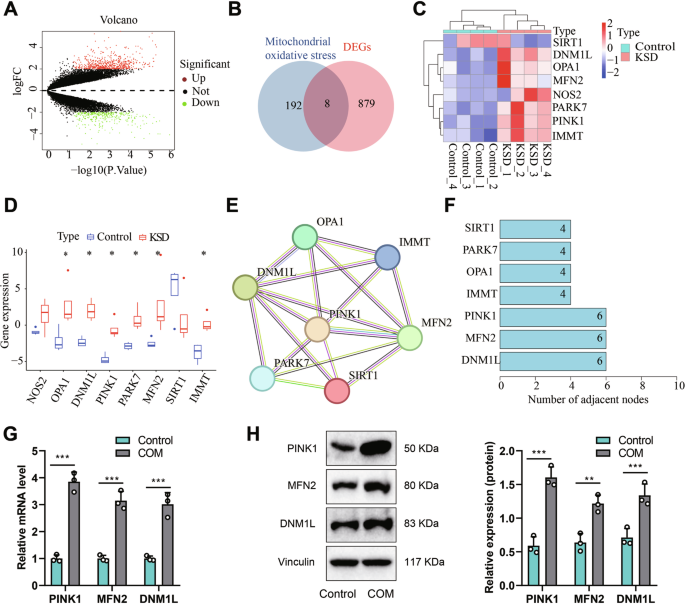
This study aims to uncover the molecular mechanisms underlying pediatric kidney stone formation induced by renal calcium deposition by utilizing high-throughput sequencing data to reveal the regulation of PINK1 by MyoD1. We performed transcriptome sequencing on peripheral blood samples from healthy children and children with kidney stones to obtain differentially expressed genes (DEGs). Genes related to mitochondrial oxidative stress were obtained from the Genecards website and intersected with DEGs to obtain candidate target genes. Additionally, we conducted protein-protein interaction (PPI) analysis using the STRING database to identify core genes involved in pediatric kidney stone disease (KSD) and predicted their transcription factors using the hTFtarget database. We assessed the impact of MyoD1 on the activity of the PINK1 promoter using dual-luciferase reporter assays and investigated the enrichment of MyoD1 on the PINK1 promoter through chromatin immunoprecipitation (ChIP) experiments. To validate our hypothesis, we selected HK-2 cells and established an in vitro kidney stone model induced by calcium oxalate monohydrate (COM). We evaluated the expression levels of various genes, cell viability, volume of adherent crystals in each group, as well as mitochondrial oxidative stress in cells by measuring mitochondrial membrane potential (Δψm), superoxide dismutase (SOD) activity, reactive oxygen species (ROS), and malondialdehyde (MDA) content. Mitochondrial autophagy was assessed using mtDNA fluorescence staining and Western blot analysis of PINK1-related proteins. Apoptosis-related proteins were evaluated using Western blot analysis, and cell apoptosis was measured using flow cytometry. Furthermore, we developed a rat model of KSD and assessed the expression levels of various genes, as well as the pathologic changes in rat renal tissues using H&E and von Kossa staining, transmission electron microscopy (TEM), and the expression of creatinine, blood urea nitrogen, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1) to evaluate the mitochondrial oxidative stress in vivo (through measurement of Δψm, SOD activity, ROS, and MDA content). Mitochondrial autophagy was evaluated by Western blot analysis of PINK1-associated proteins. Apoptosis-related proteins were detected using Western blot analysis, and cellular apoptosis was examined using cell flow cytometry and TUNEL staining. Bioinformatics analysis revealed that the PINK1 gene is upregulated and vital in pediatric kidney stone patients. Our in vitro and in vivo experiments demonstrated that silencing PINK1 could inhibit kidney stone formation by suppressing mitochondrial oxidative stress both in vitro and in vivo. We identified MyoD1 as an upstream transcription factor of PINK1 that contributes to the occurrence of pediatric kidney stones through the activation of PINK1. Our in vivo and in vitro experiments collectively confirmed that silencing MyoD1 could inhibit mitochondrial oxidative stress, mitochondrial autophagy, and cellular apoptosis in a rat model of kidney stones by downregulating PINK1 expression, consequently suppressing the formation of kidney stones. In this study, we discovered that MyoD1 may promote kidney stone formation and development in pediatric patients by transcriptionally activating PINK1 to induce mitochondrial oxidative stress.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


